Abstract
Herein, we report a photocatalyst (PC)-free, visible-light (30 W LED)-driven green and sustainable synthesis of a series of functionalized tetraketones by a one-pot multicomponent reaction at room temperature. The ethanol-mediated reaction of variably substituted aromatic/heteroaromatic, aliphatic aldehydes, and dimedone furnished the biologically relevant tetraketones in very good yields (72–92%) in 10 min to 5 h. The key advantages of the current process include its environmentally benign aspect, operational simplicity, easy isolation of the products, high yields, PC- and metal-free aspects, employment of visible light as a green and sustainable energy source, and gram-scale synthesis. The synthesized tetraketones are further subjected to in silico studies which investigate the potential of synthesized tetraketones as inhibitors targeting CHIKV nsP2 and DENV NS2B-NS3 viral proteases through a combination of virtual screening, molecular docking, and MD simulation studies. We evaluated the binding affinity and inhibitory efficacy of the synthesized tetraketones against the viral proteases: CHIKV nsp2 and DENV NS2B-NS3. Pleasingly, our findings demonstrate that compound 3f exhibits promising inhibitory activity, with binding affinities of −24.22 and −29.39 kcal/mol for CHIKV nsp2 and DENV NS2B-NS3 protease, respectively. This result highlights the potential of tetraketones as novel antiviral agents against CHIKV and DENV.
1. Introduction
Contemporary organic synthesis demands alternative synthetic approaches to minimize waste generation from traditional synthetic procedures. Green chemistry, therefore, is receiving a great deal of attention. Green chemistry mainly encourages the employment of green energy sources, nonhazardous and nontoxic chemicals, minimizing waste generation, use of green catalysts, reducing step count, etc. , In this context, several green approaches such as employing fruit juice and fruit waste as catalysts, ashes of organic waste as reagents, utilizing alternative energy sources like visible light, ultrasonication, microwave irradiation, ball milling, electrochemical approach, and grindstone chemistry have been extensively studied in recent years. Among these greener alternatives, visible light is considered a renewable, green, and abundant energy source. Many organic substances could not absorb visible light; thus, transition-metal-based photosensitizers and organophotocatalysts have been utilized which absorb light and activate the substrate by generating radicals via single electron transfer, which again raises another concern of metal contamination and additional steps for photocatalyst (PC) removal. In this backdrop, the visible-light-driven PC-free organic transformations have been less explored. The multicomponent reaction (MCR) is another green and sustainable wing in current sustainable organic synthesis. The MCR is an efficient tool for synthesizing complex molecular structures in one pot in an atom-economic manner which minimizes waste generation, overall reaction steps, and energy consumption and avoids extra workup steps. Therefore, the MCRs are greatly appreciated in the synthesis of natural products, medicinal chemistry, agrochemistry, polymer chemistry, and bioconjugation chemistry.
The tetraketones derived from cyclic 1,3-dicarbonyls are present in natural products and possess a broad spectrum of bioactivities. In particular, the tetraketones derived from the reaction of aldehyde and dimedone or cyclohexan-1,3-dione first reported in 1894 by Merling are an important class of molecules that display a broad spectrum of bioactivities such as antioxidant, antiviral, antibacterial, and enzyme inhibition activities. Also, tetraketones are promising candidates in the treatment of cancer, asthma, and inflammation. Some representative examples of dimedone-derived bioactive tetraketones are displayed in Figure . Compounds I and II show tyrosinase inhibitory activity, compounds III and IV show lipoxygenase inhibitory activity, compound V is an antifungal agent, and compounds VI and VII possess antioxidant properties.
1.
Representative examples of bioactive tetraketones.
Such an impressive biological profile of tetraketones makes them interesting candidates for medicinal chemists; thus, designing mild, green, and sustainable routes with a broad functional group tolerance is a challenging task for synthetic organic chemists. 1,3-Dicarbonyl compounds are mostly used for the synthesis of tetraketones and xanthene derivatives with the reaction of aldehyde. Several approaches have been reported over the years using various acid catalysts, metal catalysts, and metal-based nanocatalysts. Scheme shows some recent literature reports on the synthesis of tetraketones. Borah and Chowhan et al., in 2023, reported a TsOH-catalyzed synthesis of tetraketones from aromatic aldehydes and dimedone by liquid-assisted grinding (Scheme , eq a). Next, Gupta and Mozumdar, in a recent report, employed a two-step synthesis of bisimidazolium ionic liquid supported on graphene oxide in toluene and applied as a nanocatalyst for the synthesis of tetraketones from the reaction of aromatic aldehydes and dimedone (Scheme , eq b). In 2024, Kali and Maiti demonstrated the ionic liquid ([BCMIM][Cl])-mediated synthesis of tetraketones in EtOH/H2O at room temperature (Scheme , eq c). Recently, a blue LED-assisted toluene-mediated synthesis of tetraketones in the presence of eosin Y as a PC has been reported by Mondal and Ganaprakasam et al. (Scheme , eq d), where tert-amine has been used as an alkyl surrogate. All of the methods have their own advantages and novelty. Apart from these, there are several reports, − ,− where harsh reaction conditions such as high temperature, expensive metal catalysts, and specially designed catalysts have been used. Thus, a green and sustainable approach is highly desirable. In continuation of our work on natural product synthesis and sustainable methodology development, we turned our attention to developing a greener approach for the synthesis of tetraketones from readily commercially available substrates. In this report, we present a visible-light-driven PC-free one-pot multicomponent synthesis of tetraketones from readily commercially available substrates in the presence of visible light (30 W white LED) in EtOH (Scheme , eq e).
1. Comparison of Previous Works (Equation a–d) with the Present Work (Equation e).

Chikungunya virus (CHIKV) and Dengue virus (DENV) are significant global health threats and are responsible for numerous large-scale outbreaks in the last 20 years. Currently, there are no FDA-approved therapeutics for these viruses. Both CHIKV nsP2 and DENV NS2B-NS3 proteases are crucial for their replication and maturation processes. Thus, the potential of the synthesized tetraketones is further subjected to in silico studies targeting CHIKV nsP2 and DENV NS2B-NS3 viral proteases through a combination of virtual screening, molecular docking, and MD simulation studies.
2. Chemistry
2.1. Results and Discussion
The study was initiated by taking 4-nitrobenzaldehyde 2m and dimedone 1a as model substrates with the objective to develop a synthetic route at room temperature by avoiding any harsh reaction conditions and catalysts. During the literature survey, we realized that an acidic condition is required for the reaction to activate the carbonyl group of the aldehyde. Both Bronsted and Lewis acids can promote the reaction. To synthesize the tetraketones in catalyst-free conditions, we performed the first reaction of 1a (1.0 equiv) and 2m (0.5 equiv) under catalyst-free and solvent-free conditions; the reaction did not proceed, and a trace amount of product was observed (TLC-monitored) after 1 h of reaction (entry 1, Table ). Next, the same reaction was performed under catalyst-free conditions and varied solvent systems such as H2O, EtOH, DMF, DCM, and THF, resulting in 53%, 60%, 15%, 35%, and 20% yields of the products, respectively (entries 2–6, Table ). The structure of the product was confirmed by 1H and 13C NMR spectra, in agreement with the previous reports (vide ESI). In the recent years, carbonyl compound-based MCRs for the synthesis of heterocycles have been reported in the presence of visible light and the absence of any PCs. The visible light promotes the reaction, and no Lewis acid or base is required. , Thus, we hypothesized that this reaction may be promoted in the presence of visible light. Thus, we performed the reaction in EtOH/H2O in the presence of a 30 W white LED. To our delight, the reaction proceeded smoothly, and 78% yield of the product was isolated within 40 min of the reaction (entry 7, Table ). Next, the same reaction was performed in the presence of only EtOH as the solvent, and pleasingly 91% yield of tetraketone 3m was isolated in 40 min (entry 8, Table ). To check the effect of light intensity on the reaction rate, the next reaction was performed in the presence of 9 W and 12 W LED, which resulted in 63% and 72% yields of the tetraketone 3m, respectively, at the same reaction time (entries 9–10, Table ). Also, to check the necessity of light, a reaction was performed in dark condition, which resulted in 38% yield of 3m after 40 min (entry 11, Table ). Further, to check the efficacy of the method in different solvents in the presence of LED, various solvents such as DCM, CHCl3, DMF, and THF were screened, and no solvent gave better results than EtOH (entries 12–15, Table ). From the above findings, it was revealed that EtOH provides best results for the transformation in a shorter time. Next, reactions in the presence of both light and acid catalysts were performed to check for any significant increase in the yield. In the presence of molecular iodine and sulfamic acid also, no significant increase in the yield was observed (entries 16–17, Table ). Thus, the reaction in EtOH in the presence of 30 W LED under catalyst-free conditions at room temperature was found to be the optimized condition providing tetraketone 3m (entry 8, Table ).
1. Optimization of the Reaction Conditions .
| entry | solvent | catalyst | temp. (°C) | time (min) | yield (%) |
|---|---|---|---|---|---|
| 1 | solvent-free | catalyst-free | RT | 60 | trace |
| 2 | H2O | catalyst-free | RT | 60 | 53 |
| 3 | EtOH | catalyst-free | RT | 60 | 60 |
| 4 | DMF | catalyst-free | RT | 60 | 15 |
| 5 | DCM | catalyst-free | RT | 60 | 35 |
| 6 | THF | catalyst-free | RT | 60 | 20 |
| 7 | EtOH/H2O | catalyst-free | RT | 40 | 78 |
| 8 | EtOH | catalyst-free | RT | 40 | 91 |
| 9 | EtOH | catalyst-free | RT | 40 | 63 |
| 10 | EtOH | catalyst-free | RT | 40 | 72 |
| 11 | EtOH | catalyst-free | RT | 40 | 38 |
| 12 | DCM | catalyst-free | RT | 60 | 50 |
| 13 | CHCl3 | catalyst-free | RT | 60 | 52 |
| 14 | DMF | catalyst-free | RT | 60 | 55 |
| 15 | THF | catalyst-free | RT | 60 | 30 |
| 16 | EtOH | I2 (20) | RT | 40 | 90 |
| 17 | EtOH | SA (20) | RT | 40 | 91 |
Reaction conditions: 1a (1.0 mmol) and 2m (0.5 mmol) in 0.5 mL solvent.
Isolated yield.
The reaction was performed in the presence of 30 W LED.
The reaction was performed in the presence of 9 W LED.
The reaction was performed in the presence of 12 W LED.
The reaction was performed in dark.
With the optimized reaction conditions in hand, we turned our attention to study the generality of the reaction. Thus, the reaction of benzaldehydes with electron-donating substituents such as para-anisaldehyde 2a, 4-methylthio benzaldehyde 2b, cuminaldehyde 2c, 3,4-dimethylbenzaldehyde 2d, 3-hydroxy-4-methoxybenzaldehyde 2e, and 3,4,5-trimethoxybenzaldehyde 2f with dimedone 1a resulted in the corresponding tetraketones 3a, 3b, 3c, 3d, 3e, and 3f in 83%, 82%, 81%, 82%, 87%, and 91% yields, respectively (Scheme ). Next, to check the steric effect of the substituents on benzaldehyde, 2-methoxybenzaldehyde 2g and 2-methylbenzaldehyde 2h were reacted with dimedone, and the corresponding tetraketones 3g and 3h were isolated in 84% and 82% yields, indicating that steric crowding has no significant effect on the product yield. Furthermore, halogen substituents on benzaldehyde were studied by reacting 4-fluorobenzaldehyde 2i, 4-chlorobenzaldehyde 2j, and 3-bromobenzaldehyde 2k with dimedone 1a. The respective haloarene-linked tetraketones 3i, 3j, and 3k were isolated in 87%, 85%, and 81% yields, respectively. To check the effect of electron-withdrawing groups on benzaldehyde, 4-formylbenzonitrile 2l and 4-nitrobenzaldehyde 2m were treated with dimedone 1a, and gratifyingly, 86% and 91% yields of the corresponding tetraketones 3l and 3m were obtained. Sterically hindered α-naphthaldehyde 2n also reacted smoothly to provide 3n in a 72% yield. The scope of heteroaromatic aldehyde was also screened by using thiophene-2-carboxaldehyde 2o to synthesize the corresponding tetraketone 3o in 76% yield. The scope of formaldehyde was checked by reacting formaldehyde 3p with dimedone 1a and cyclohexane-1,3-dione 1b, and the respective methylene-bridged tetraketones 3p and 3q were isolated in 92% and 74% yields.
2. Substrate Scope for Visible-Light-Driven Synthesis of Tetraketones .
a Reaction conditions: Compound 1 (1.0 mmol) and compound 2 (0.5 mmol) in 0.5 mL EtOH were taken in a 5 mL reaction tube and stirred at room temperature in the presence of 30 W LED until completion of the reaction (TLC monitored).
To check whether the present method is applicable for the synthesis of tetraketones on a large scale, a representative reaction was performed by taking 4-nitrobenzaldehyde 2m (4.0 mmol) and dimedone 1a (8.0 mmol) under optimized conditions in 4.0 mL of ethanol. Pleasingly, the corresponding product 3m was isolated in 88% yield (1.4 g), which accounts for the practicality of the method (Scheme ).
3. Gram-Scale Synthesis of 3m .
The plausible reaction pathway is proposed in accordance with previously reported literature , on radical mechanism, as shown in Scheme . In the presence of visible light, the photoactivation of aldehyde generates the radical intermediate 4, which in turn abstracts hydrogen from dimedone 1a, generating the radical intermediates 5 and 6. Next, the radical combination of benzyl radical 5 and dimedone radical 6 resulted in β-hydroxyketone (an aldol-type adduct) 7, which undergoes dehydration, forming the Michael acceptor 8. The subsequent Michael addition of 1a with 8 formed the desired tetraketone 3m.
4. Plausible Reaction Pathway.
2.2. Comparison
Owing to the promising bioactivities and pharmaceutical properties of tetraketones, several methods have been reported over the years. Table shows the comparison of the present work with the previously reported works. − It is evident from the table that the present method provides a greener and sustainable approach for the synthesis of medicinally relevant tetraketones, which proceeds under catalyst-free conditions at room temperature under visible light (30 W white LED), by avoiding the use of any expensive, metal-based catalysts, specially designed catalysts, and any harsh reaction conditions.
2. Comparison of the Present Work with the Previous Reports.
| Sl. No. | catalyst | reaction conditions | time | yield (%) | refs |
|---|---|---|---|---|---|
| 01 | DDIS@PS | H2O or EtOH, RT | 1.5–6.5 h | 65–94 | |
| 02 | Fe3O4@SiO2@Ni–Zn–Fe LDH | H2O, reflux | 2–40 min | 89–96 | |
| 03 | Pd(0)-EDA/SC-2 | H2O, 100 °C | 1.2 h | 82–90 | |
| 04 | SmCl3 | H2O, RT | 15–30 min | 82–97 | |
| 05 | C/TiO2–SO3H | H2O, 100 °C | 0.25–5 h | 88–94 | |
| 06 | GO@ANSA | EtOH, reflux | 20–45 min | 88–97 | |
| 07 | GO/ZnO nanorods | H2O, reflux | 10–15 min | 60–99 | |
| 08 | CNF@DBU[Cl] nanofibers | H2O, RT | 2–65 min | 30–96 | |
| 09 | nano BiOCl | H2O, 35 °C, ultrasound | 12–17 min | 83–95 | |
| 10 | nano ZnS | H2O, RT | 30–50 min | 85–97 | |
| 11 | Ni(0) nanoparticles | solvent-free, MW (850 W) | 5–20 min | 75–95 | |
| 12 | nanoCuFe2O4@SO3H | EtOH, RT | 45–75 min | 82–97 | |
| 13 | nano Fe/NaY zeolite | H2O, reflux | 20 min-2 h | 90–98 | |
| 14 | I2 | H2O, 100 °C | 20–30 min | 89–97 | |
| 15 | catalyst-free | 30 W LED, EtOH, RT | 10 min-5 h | 72–92 | this work |
3. In Silico Analysis
3.1. Materials and Methods
Available in the Supporting Information.
3.2. Drug-Likeness Properties through ADMET
Drug-likeness prediction was initially performed to assess the pharmacokinetic properties and potential oral bioavailability of tetraketone derivatives targeting CHIKV nsP2 protease and DENV NS2B-NS3 protease. Lipinski’s rule of five and other relevant drug-likeness filters were applied to screen a library of compounds, resulting in the selection of 12 tetraketone derivatives deemed suitable for further investigation like molecular docking, as has been shown in Table 1 in the Supporting Information.
3.3. Molecular Docking
Molecular docking studies were conducted to evaluate the binding affinity and interactions of the selected tetraketone derivatives with both the nsP2 protease of CHIKV and the NS2B-NS3 protease of DENV. Then, the compounds were docked into the active site of both proteases using computational tools such as AutoDock Vina. Docking scores ranging from −5.0 to −6.1 kcal/mol indicated favorable binding interactions for several compounds.
Among the top-ranked compounds, tetraketones 3c and 3f demonstrated the highest docking score of −5.7 and −5.8 kcal/mol, respectively, for CHIKV nsp2 protease and −6.1 and −5.4 kcal/mol, respectively, for DENV NS2B-NS3 protease, as shown in Table .
3. Binding Affinities and Interacting Residues of All Four Complexes (CHIKV nsP2 Pro-3c, CHIKV nsP2 Pro-3f, DENV NS2B-NS3 Pro-3c, and DENV NS2B-NS3 Pro-3f) Derived from Molecular Docking Studies through AutoDock Vina Tool.
| complex | binding affinity (kcal/mol) | interacting residues |
|---|---|---|
| CHIKV nsP2 Pro-3c | –5.7 | SER482, HIS548, TRP549, ASN551 |
| CHIKV nsP2 Pro-3f | –5.8 | HIS548, TRP549, ASN551 |
| DENV NS2B-NS3 Pro-3c | –6.1 | HIS51, LYS74, GLY87, SER135, GLY151 |
| DENV NS2B-NS3 Pro-3f | –5.4 | HIS51, LYS74, GLY133, GLY151 |
3.4. Molecular Dynamics Simulation Studies
Molecular dynamics simulations of the selected tetraketone derivatives in complex with CHIKV and DENV proteases over 20 ns demonstrated the stability of the protein-ligand complexes. Trajectory analyses like root-mean-square deviation (RMSD), root-mean-square fluctuation (RMSF), and radius of gyration (ROG) indicated that the two tetraketone derivatives, i.e., 3c and 3f, maintained stable binding modes throughout the simulation period against both CHIKV nsP2 and DENV NS2B-NS3 proteases.
3.5. Root-Mean-Square Deviation (RMSD)
The RMSD values for the tetraketone derivatives complexed with both CHIKV nsP2 protease and DENV NS2B-NS3 protease, specifically compound 3f, remained relatively stable around 1.35 and 2.62 Å, , respectively, throughout the 20 ns simulation period as compared to 3c, as shown in Table and Figure . This stability indicates that the 3f–CHIKV protease complex is well-maintained, with minimal deviations from the initial binding pose.
4. RMSD, RMSF, and ROG Values of CHIKV nsP2 and DENV NS2B-NS3 Proteases Complexed with Tetraketone Derivatives .
| RMSD
(Å) |
RMSF
(Å) |
ROG
(Å) |
||||
|---|---|---|---|---|---|---|
| name of complex | AVG | STDEV | AVG | STDEV | AVG | STDEV |
| CHIKV nsP2-3c | 1.0995 | 0.1426 | 0.9888 | 0.3888 | 20.4888 | 0.0878 |
| CHIKV nsP2-3f | 1.3536 | 0.1778 | 1.0610 | 0.4323 | 20.6659 | 0.1159 |
| DENV NS2B-NS3-3c | 1.9683 | 0.2715 | 1.2301 | 0.9252 | 16.5274 | 0.1189 |
| DENV NS2B-NS3-3f | 2.6205 | 0.3624 | 1.4509 | 1.3772 | 16.9838 | 0.1756 |
All values are given in Å.
2.
RMSD trajectories of all four complexes of tetraketone derivative–protease complexes over the simulation period of 20 ns.
Similarly, in the case of DENV protease, the plot also showed that tetraketone 3f demonstrated a stable complex throughout the 20 ns simulation period with DENV protease compared to 3c.
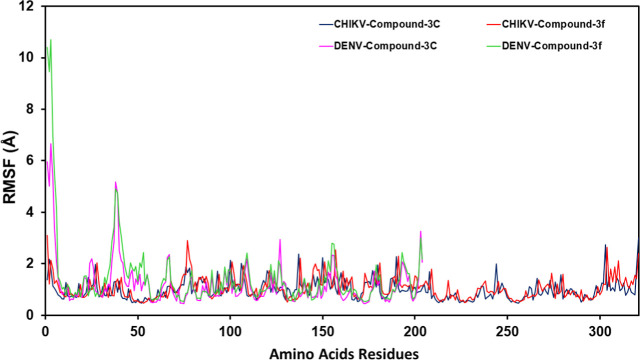
3.6. Root-Mean-Square Fluctuation (RMSF)
The RMSF analysis provided insights into the flexibility of the protein residues in the presence of tetraketone derivatives (3c and 3f), and the RMSF plots and values for both compounds against DENV and CHIKV protease are shown in Figure and mentioned in Table . The RMSF analysis revealed that the binding of 3f resulted in reduced fluctuations in the active site residues, particularly in the catalytic region (Cys478 and His548). RMSF values for these residues were significantly lower compared to those of the unbound protease, suggesting that 3f stabilizes the CHIKV nsP2 active sites. Tetraketone 3c also showed reduced fluctuations but to a lesser extent compared to 3c. In the case of DENV protease, 3f caused a marked reduction in RMSF values in the active site region (His51, Ser135), indicating effective stabilization of the protease. Tetraketone 3c also showed reduced RMSF in these regions, though not as pronounced as 3f.
3.
RMSF trajectories of the CHIKV and DENV protease amino acid residues in the presence of 3c and 3f. The plot shows the fluctuation of each residue during MD simulations. The blue line represents CHIKV nsP2 in the presence of 3c; the red line represents CHIKV nsP2 protease in the presence of 3f; the pink line represents DENV NS2B-NS3 protease in the presence of 3c; and green line represents DENV NS2B-NS3 protease in the presence of 3f. High RMSF values indicate increased flexibility in the loop regions and terminal regions.
3.7. Radius of Gyration (ROG)
The ROG analysis was performed to understand the compactness of tetraketone derivative–protease complexes. The ROG of the CHIKV nsP2 protease–tetraketone derivative complex remained fairly constant at approximately 20.5 Å, indicating that the binding of the tetraketone derivative did not significantly alter the protein’s overall structure (Figure ). Similarly, the ROG for the DENV NS2B-NS3 protease–tetraketone derivative complex was stable around 16.9 Å, suggesting that ligand binding did not induce substantial conformational changes in the protein structure (Figure ).
4.
ROG trajectories of all four complexes of tetraketone derivative–protease complexes over the simulation period of 20 ns.
3.8. Binding Free Energy Calculation
The MM/PBSA.py tool, first designed for the AMBER software, is widely used for computing absolute binding affinities at a moderate computational effort. Nowadays, Molecular Mechanics-Generalized Born Surface Area (MM/GBSA) is employed in the majority of instances due to its faster calculation rate and relatively greater accuracy. In this study, binding free energies were calculated using MM-GBSA methods to quantify the interaction strength of complex tetraketone derivatives with both the DENV and CHIKV proteases using the Amber 18 package. The binding free energies of all 4 complexes, i.e., 3c_CHIKV nsp2 protease, 3f_CHIKV protease, 3c_DENV NS2B-NS3 protease, and 3f_DENV NS2B-NS3 protease, are mentioned in Table . In our previous studies on HIV-1 protease, − SARS-CoV-2 Mpro, and DENV NS2B-NS3 protease − systems, we utilized the MM-PB/GBSA approach, which we repeated here.
5. Binding Free Energy Components for Both the CHIKV nsP2 and DENV NS2B-NS3 Proteases Complexed with Tetraketone Derivatives, Computed from 500 Snapshots through MM-GBSA .
| energy
components |
|||||||
|---|---|---|---|---|---|---|---|
| protein (receptor) | tetraketone derivatives (ligand) | ΔE vdw | ΔE ele | ΔG pol | ΔG nonpol | ΔG Total | |
| CHIKV nsP2 Pro | 3c | AVG | –25.0056 | –3.9768 | 10.2266 | –2.6412 | –21.3971 |
| SD | 4.3016 | 4.8415 | 4.5578 | 0.4706 | 3.7083 | ||
| 3f | AVG | –30.0431 | –2.9538 | 11.705 | –2.9296 | –24.2215 | |
| SD | 3.4509 | 1.9362 | 2.1816 | 0.2957 | 2.9797 | ||
| DENV NS2B-NS3 Pro | 3c | AVG | –37.0733 | –9.6038 | 22.0866 | –4.1575 | –28.748 |
| SD | 2.9428 | 4.9863 | 5.3151 | 0.2583 | 2.6377 | ||
| 3f | AVG | –36.0363 | –1.781 | 12.6947 | –4.2725 | –29.3951 | |
| SD | 4.3016 | 4.8415 | 4.5578 | 0.4706 | 3.7083 | ||
All energy component values are given in kcal/mol. Components: E vdw, van der Waals energy; E ele, electrostatic energy in the gas phase; Gnon-pol, nonpolar solvation energy; ΔGpol, polar solvation energy. .
The MM-GBSA binding free energies for tetraketones 3c and 3f with CHIKV nsP2 protease were calculated to be −21.39 kcal/mol and −24.22 kcal/mol, respectively, while the binding free energy values of 3c and 3f with DENV NS2B-NS3 protease were −28.74 kcal/mol and −29.39 kcal/mol, respectively, as shown in Figure . For both CHIKV and DENV, the major contributors to the binding free energy were found to be van der Waals interactions and electrostatic interactions. These results suggest that, in both the cases of CHIKV and DENV, the binding free energy of 3f is higher as compared to that of 3c. The calculated binding energies are consistent with the observed stability and low RMSD values, indicating that 3f is likely to exhibit an effective inhibitory activity against both proteases.
5.
Binding free energy calculations for tetraketone derivative–protease complexes using the MM-GBSA method.
3.9. Hotspot Residue Interaction Analysis
The interaction analysis of hotspot residues revealed significant changes in binding interactions between CHIKV nsP2 and DENV NS2B-NS3 proteases and 3c and 3f of tetraketone derivatives, as shown in Figures and and detailed values are mentioned in Table . For CHIKV nsp2 protease, key hotspot residues such as Pro485, Leu539, and Asn551 exhibited enhanced interaction frequencies with 3c, and key residues like Val540, Ser541, Asn551, Arg552, and Pro553 interact with 3f. These residues are known to play crucial roles in the binding and stabilization of ligands (Table ).
6.
Hotspot residues identified in the CHIKV nsP2 protease–tetraketone derivative complexes.
7.
Hotspot residues identified in the DENV NS2B-NS3 protease–tetraketone derivative complexes.
6. Hotspot Residues in the Interactions of CHIKV nsP2 and DENV NS2B-NS3 Proteases with Tetraketone Derivatives .
| hotspot residues | E vdw | E ele | G pol | G nopol | G side_chain | G backbone | G Total |
|---|---|---|---|---|---|---|---|
| CHIKV-nsP2 pro-3c | |||||||
| PRO485 | –1.763 | 1.093 | 0.048 | 0.022 | –1.391 | –0.290 | –1.681 |
| LEU539 | –1.190 | 1.603 | 0.071 | –0.074 | –0.988 | –0.066 | –1.054 |
| ASN551 | –1.818 | 1.498 | 0.067 | –0.649 | –1.047 | –0.177 | –1.224 |
| CHIKV-nsP2 pro-3f | |||||||
| VAL540 | –2.087 | 0.773 | 0.035 | –0.064 | –1.436 | –0.130 | –1.567 |
| SER541 | –1.648 | 0.818 | 0.037 | –0.183 | –0.815 | –0.562 | –1.378 |
| ASN551 | –2.660 | 0.700 | 0.032 | –0.105 | –1.034 | –0.335 | –1.370 |
| ARG552 | –1.479 | 0.733 | 0.033 | 0.647 | –0.964 | –0.164 | –1.129 |
| PRO553 | –2.225 | 0.866 | 0.040 | –0.103 | –1.963 | –0.534 | –2.4985 |
| DENV-NS2B-NS3 Pro-3c | |||||||
| HIS51 | –2.169 | 0.915 | 0.045 | –0.546 | –1.679 | –0.094 | –1.773 |
| LEU128 | –1.629 | 0.632 | 0.031 | –0.234 | –1.608 | –0.064 | –1.672 |
| PRO132 | –1.979 | 0.381 | 0.018 | –0.160 | –1.636 | –0.618 | –2.255 |
| ASN152 | –1.073 | 0.420 | 0.020 | –0.954 | –0.525 | –0.704 | –1.229 |
| GLY153 | –1.788 | 0.319 | 0.015 | –1.001 | –0.444 | –1.297 | –1.741 |
| TYR161 | –1.842 | 0.871 | 0.043 | –0.542 | –1.036 | –0.085 | –1.121 |
| DENV-NS2B-NS3 Pro-3f | |||||||
| HIS51 | –2.338 | 1.153 | 0.051 | –1.297 | –1.927 | –0.159 | –2.086 |
| LEU128 | –1.216 | 0.482 | 0.021 | –0.114 | –1.263 | –0.057 | –1.320 |
| PRO132 | –2.852 | 0.779 | 0.034 | –0.350 | –2.293 | –0.684 | –2.977 |
| TYR161 | –1.954 | 0.805 | 0.036 | –0.112 | –1.724 | –0.002 | –1.726 |
Only residues of ΔG values ≥1.0 kcal/mol were taken into consideration.
In addition, for DENV NS2B-NS3 protease, key hotspot residues like His51, Leu128, Pro132, Asn152, Gly153, and Tyr161 interact with tetraketone 3c, and hotspot residues such as His51, Leu128, Pro132, and Tyr161 interact with 3f. The increased interaction frequencies suggest that tetraketone derivatives form strong and specific interactions with these residues, potentially contributing to their efficacy as inhibitors.
3.10. Receptor–Ligand Interactions
A comprehensive interaction summary of residues regarding conventional H-bonds, C–H bonds, van der Waals interactions, water hydrogen bonds, alkyl, and pi–alkyl was also calculated using the Biovia Discovery Studio V21.1.0.20298 software package (http://www.3dsbiovia.com) and is shown in Figure . It specifies that many of the residues present precisely in the active site pocket and nearby this region play an important role in the evolution of H bonds via these synthesized compounds. Biovia Discovery Studio software calculated the individual system’s residues’ H bonds and van der Waals interactions. Here, the residues like Leu539, Val540, Phe560, and Pro562 (CHIKV nsP2-3c complex); Pro485, Val540, Ser541, and Pro553 (CHIKV nsP2-3f complex); Met49, His51, Leu128, Pro132, Tyr150, and Tyr161 (DENV NS2B-NS3pro-3c complex); and His03, Leu128, Pro132, Ser135, Gly153, and Tyr161 (DENV NS2B-NS3pro-3f complex) are found near the active site pocket having H bonds, alkyl, and pi–alkyl with the compounds and play an important role in the interaction. Aside from H bonds, alkyl, and pi–alkyl, many residues in the active site or its surroundings contribute via van der Waals interactions, as shown in all four complexes in Figure . The binding interactions of the target proteins (DENV NS2B-NS3 protease and CHIKV nsP2 protease) with the drug candidates (3c and 3f) after 20 ns are shown in Figure , which correlate with the bonding as shown in Figure .
8.
MD simulation analyses of both the screened synthetic compounds with the target proteins (DENV NS2B-NS3 protease and CHIKV nsP2 protease) revealed the expected binding modes on average with the drug candidates (3c and 3f). Ligands/compounds are depicted as gray stick models, while interaction residues are depicted as dyed spheres. Green spheres represent conventional H bonds; light green spheres represent C–H bonds; light pink spheres represent alkyl and pi–alkyl interactions; purple spheres represent pi–sigma interactions; and aqua blue spheres represent water hydrogen bonds.
9.
Binding interactions of the target proteins (DENV NS2B-NS3 protease and CHIKV nsP2 protease) with the drug candidates (3c and 3f) after 20 ns. Ligands/compounds and residues are depicted as stick models: (A) CHIKV nsP2 protease/3c complex, (B) CHIKV nsP2 protease/3f complex, (C) DENV NS2B-NS3 protease/3c complex, and (D) DENV NS2B-NS3 protease/3f complex.
3.11. Natural Bond Orbital Analysis: Hydrogen Bonds
Natural bond orbital (NBO) analysis is a computational chemistry technique that describes the bonding structure of compounds, which interprets the electron density distribution in a molecule in terms of chemical bonds and lone pairs, providing a more intuitive understanding of molecular bonding than other quantum chemical approaches. One of the key aspects of NBO analysis is donor–acceptor interactions, which include the hydrogen bonds (H bonds). In the current study, we have analyzed the detailed H bonding of the ligands (3c and 3f) with the target proteins (CHIKV nsP2 and DENV NS2B-NS3) to interpret their interactions.
H-bond analysis was conducted to assess the stability of the ligand–protein interactions based on hydrogen-bonding patterns. In the case of CHIKV, compound 3c exhibited a higher number of stable H bonds with CHIKV nsP2 protease throughout the 20 ns simulation, which indicated stronger and more persistent interactions between the compound and the protein. This suggests compound 3c forms favorable and consistent H bonds, as shown in Figure , contributing to a more stable binding. In contrast, tetraketone 3f showed fewer and fewer consistent H bonds during the simulation, indicating weaker interactions and a more dynamic binding mode. In the case of DENV, 3c and 3f formed a significant number of H bonds with DENV NS2B-NS3 protease throughout the 20 ns simulation, as shown in Figure . This indicates that both compounds can establish stable and reliable H bonds with DENV protease, supporting their potential as effective inhibitors. The H-bond network was relatively similar for both compounds, suggesting that both derivatives exhibit comparable binding affinities in DENV protease.
10.
Hydrogen-bond interaction analysis of 3c and 3f with CHIKV nsP2: (a) CHIKV nsP2 with 3c and (b) CHIKV nsP2 with 3f.
11.
Hydrogen-bond interaction analysis of 3c and 3f with DENV NS2B-NS3: (a) DENV NS2B-NS3 with 3c and (b) DENV NS2B-NS3 with 3f.
4. Conclusions
In summary, a visible-light-driven PC-free one-pot multicomponent green sustainable synthesis of the functionalized tetraketone scaffolds has been reported at room temperature in EtOH. The present approach offers several advantages such as no use of any metal-based or organophotocatalysts, high yield of the products without column chromatographic purification, and gram-scale synthesis of tetraketones. The study also investigates the potential of the synthesized tetraketones as inhibitors targeting CHIKV nsP2 protease and DENV NS2B-NS3 protease through a combination of virtual screening, molecular docking, and MD simulation. Overall, the results of this study indicate that tetraketones exhibit significant stabilizing effects on both CHIKV nsP2 protease and DENV NS2B-NS3 protease, as evidenced by reduced RMSD and RMSF values, more compact protein structures, as shown by ROG, and suitable binding interactions with hotspot residues. The binding free energy calculations further support the effectiveness of these compounds as potential inhibitors. These findings provide valuable insights into the design of tetraketones as targeted antiviral agents against CHIKV nsP2 and DENV NS2B-NS3 proteases; however, further in vivo and in vitro studies are necessary to establish their efficacy as potential antiviral agents.
5. Experimental Section
5.1. General Information
All the reagents and chemicals were procured from commercial sources like Sigma Aldrich and TCI. Unless otherwise mentioned, all the chemicals were used without further purification. The reactions were monitored by thin layer chromatography (TLC). The NMR spectra (1H and 13C) were recorded on Bruker 400 and 500 MHz NMR spectrometers in CDCl3 or DMSO-d 6, with tetramethylsilane as the internal standard. Chemical shifts expressed as δ values and coupling constants as J values were reported in ppm and Hz, respectively.
5.2. General Procedure for the Synthesis of Tetraketones
A reaction tube containing a magnetic stir bar was charged with p-anisaldehyde 1a (0.5 mmol) and dimedone 2 (1.0 mmol), and 0.5 mL of EtOH was added into the reaction tube. A 30 W white LED was set near the reaction tube (as shown in the Figure S1 in the Supporting Information below). The reaction was allowed to stir under the visible light. After completion of the reaction (TLC monitor), the precipitate obtained was filtered off and washed with 3 mL aqueous ethanol and dried. Other tetraketones 3b–3q were prepared using similar methods.
Supplementary Material
Acknowledgments
The authors acknowledge Berhampur University, Bhanja Bihar for infrastructure and support. B.B.P. is grateful to SERB (SRG/2019/002032), New Delhi, India for generous funding. S.S.A. acknowledges CSIR, New Delhi, India for the Junior Research Fellowship (JRF). J.D. is grateful to UGC, New Delhi, India for the Junior Research Fellowship (JRF), and A.G. is grateful to Berhampur University for University Research Fellowship. B.R.M. is thankful to S & T Department Project-Odisha (ST-SCST-0061/2018/1288). UGC (F. 30-484/2019) (BSR) is acknowledged for financial assistance. CAIF, IISER Berhampur is greatly acknowledged for providing NMR and HRMS facilities. The authors also thank Prof. Himanshu Sekhar Biswal (NISER Bhubaneswar) and Dr. Thirupathi Barla (IISER Berhampur) for their timely help.
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.4c10660.
Sample characterization data and spectra (1H, and 13C NMR, HRMS), materials and methods (in silico), and green parameters (PDF)
∥.
S.S.A. and J.D. contributed equally.
The authors declare no competing financial interest.
References
- a Zimmerman J. B., Anastas P. T., Erythropel H. C., Leitner W.. Designing for a green chemistry future. Science. 2020;367:397–400. doi: 10.1126/science.aay3060. [DOI] [PubMed] [Google Scholar]; b Anastas P., Eghbali N.. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010;39:301–312. doi: 10.1039/B918763B. [DOI] [PubMed] [Google Scholar]; c Kitanosono T., Masuda K., Xu P., Kobayashi S.. Catalytic Organic Reactions in Water toward Sustainable Society. Chem. Rev. 2018;118:679–746. doi: 10.1021/acs.chemrev.7b00417. [DOI] [PubMed] [Google Scholar]; d Kar S., Sanderson H., Roy K., Benfenati E., Leszczynski J.. Green Chemistry in the Synthesis of Pharmaceuticals. Chem. Rev. 2022;122:3637–3710. doi: 10.1021/acs.chemrev.1c00631. [DOI] [PubMed] [Google Scholar]; e Acharya S. S., Patra S., Maharana R., Dash M., Barad L. M., Parida B. B.. Recent advances in spirocyclization of maleimides via transition-metal catalyzed C–H activation. Org. Biomol. Chem. 2024;22:2916–2947. doi: 10.1039/D3OB01904G. [DOI] [PubMed] [Google Scholar]
- a de Marco B. A., Rechelo B. S., Totoli E. G., Kogawa A. C., Salgado H. R. N.. Evolution of green chemistry and its multidimensional impacts: A review. Saudi Pharm. J. 2019;27:1–8. doi: 10.1016/j.jsps.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Swaraj Acharya S., Bhusan Parida B.. Humic Acid: A Promising and Green Bioorganic Catalyst in Organic Syntheses. ChemistrySelect. 2024;9:e202305233. doi: 10.1002/slct.202305233. [DOI] [Google Scholar]; c Acharya S. S., Patra S., Barad L. M., Roul A., Parida B. B.. Recent advances in iodine–DMSO mediated C(sp3)–H functionalization of methyl-azaarenes via Kornblum oxidation. New J. Chem. 2024;48:7614–7638. doi: 10.1039/D3NJ05359H. [DOI] [Google Scholar]; d Acharya S. S., Das B., Parida B. B.. Aminonaphthoquinone: A Versatile Synthon for the Synthesis of Naphthoquinone-fused N-heterocycles via Multicomponent Reactions (MCRs) Am. J. Heterocycl. Chem. 2025;10:26–40. doi: 10.11648/j.ajhc.20251002.11. [DOI] [Google Scholar]
- Hamidinasab M., Ahadi N., Bodaghifard M. A., Brahmachari G.. Sustainable and Bio-Based Catalysts for Multicomponent Organic Synthesis: An Overview. Polycycl. Aromat. Compd. 2023;43:5172–5226. doi: 10.1080/10406638.2022.2097278. [DOI] [Google Scholar]
- Venkateswarlu K.. Ashes from organic waste as reagents in synthetic chemistry: a review. Environ. Chem. Lett. 2021;19:3887–3950. doi: 10.1007/s10311-021-01253-4. [DOI] [Google Scholar]
- a Sun V., Lv Q.-Y., Chen X.-L., Qu L.-B., Yu B.. Recent advances in visible-light-mediated organic transformations in water. Green Chem. 2021;23:232–248. doi: 10.1039/D0GC03447A. [DOI] [Google Scholar]; b Uygur M., Mancheno O. G.. Visible light-mediated organophotocatalyzed C–H bond functionalization reactions. Org. Biomol. Chem. 2019;17:5457–5489. doi: 10.1039/C9OB00834A. [DOI] [PubMed] [Google Scholar]; c Sivanandan S. T., Jesline M. J., Nair D. K., Kumar T.. Visible Light-Mediated Reactions of β-Nitroalkenes. Asian J. Org. Chem. 2023;12:e202200555. doi: 10.1002/ajoc.202200555. [DOI] [Google Scholar]; d Tripathy A. R., Kumar A., Rahmathulla A R., Jha A. K., Yatham V. R.. Visible-Light-Driven α-Aminoalkyl Radical-Mediated C(sp3)–C(sp) Cross-Coupling of Iodoalkanes and Alkynyl Bromides. Org. Lett. 2022;24:5186–5191. doi: 10.1021/acs.orglett.2c02018. [DOI] [PubMed] [Google Scholar]; e Bisoyi A., Tripathy A. R., Yedase G. S., P S. S., Choudhury U., Yatham V. R.. Photoinduced Decarboxylative C3–H Alkylation of Quinoxalin-2(1H)-ones. J. Org. Chem. 2023;88:2631–2641. doi: 10.1021/acs.joc.2c02823. [DOI] [PubMed] [Google Scholar]
- Banerjee B.. Recent developments on ultrasound assisted catalyst-free organic synthesis. Ultrason. Sonochem. 2017;35:1–14. doi: 10.1016/j.ultsonch.2016.09.023. [DOI] [PubMed] [Google Scholar]
- Khanna A., Dubey P., Sagar R.. Exploiting Microwave-Assisted Organic Synthesis (MAOS) for Accessing Bioactive Scaffolds. Curr. Org. Chem. 2021;25:2378–2456. doi: 10.2174/1385272825666210531103927. [DOI] [Google Scholar]
- Roy K., Sahoo S., Saha A., Adak L.. Ball Milling in Organic Transformations. Curr. Org. Chem. 2023;27:153–165. doi: 10.2174/1385272827666221223143844. [DOI] [Google Scholar]
- a Karmakar I., Brahmachari G.. Electrochemical and mechanochemical synthesis of dihydrofuro[3,2-c]chromenones via intramolecular Csp3–H cross-dehydrogenative oxygenation within warfarin frameworks: an efficient and straightforward dual approach. Green Chem. 2022;24:2825–2838. doi: 10.1039/D2GC00146B. [DOI] [Google Scholar]; b Alam T., Rakshit A., Dhara H. N., Palai A., Patel B. K.. Electrochemical Amidation: Benzoyl Hydrazine/Carbazate and Amine as Coupling PartnersClick to copy article link. Org. Lett. 2022;24:6619–6624. doi: 10.1021/acs.orglett.2c02626. [DOI] [PubMed] [Google Scholar]
- Banerjee M., Panjikar P. C., Das D., Iyer S., Bhosle A. A., Chatterjee A.. Grindstone chemistry: A “green” approach for the synthesis and derivatization of heterocycles. Tetrahedron. 2022;112:132753. doi: 10.1016/j.tet.2022.132753. [DOI] [Google Scholar]
- Nazeef M., Shivhare K. N., Ali S., Ansari K., Ansari M. D., Tiwari S. K., Yadav V., Siddiqui I. R.. Visible-light-promoted C–N and C–S bonds formation: A catalyst and solvent-free photochemical approach for the synthesis of 1,3-thiazolidin-4-ones. J. Photochem. Photobiol., A. 2020;390:112347. doi: 10.1016/j.jphotochem.2019.112347. [DOI] [Google Scholar]
- Tavakolian M., Hosseini-Sarvari M.. Catalyst-Free Organic Transformations under Visible-Light. ACS Sustainable Chem. Eng. 2021;9:4296–4323. doi: 10.1021/acssuschemeng.0c06657. [DOI] [Google Scholar]
- a Zhi S., Ma X., Zhang W.. Consecutive multicomponent reactions for the synthesis of complex molecules. Org. Biomol. Chem. 2019;17:7632–7650. doi: 10.1039/C9OB00772E. [DOI] [PubMed] [Google Scholar]; b Slobbe P., Ruijter E., Orru R. V. A.. Recent applications of multicomponent reactions in medicinal chemistry. Chem. Med. Commun. 2012;3:1189–1218. doi: 10.1039/c2md20089a. [DOI] [Google Scholar]; c John S. E., Gulati S., Shankaraiah N.. Recent advances in multi-component reactions and their mechanistic insights: a triennium review. Org. Chem. Front. 2021;8:4237–4287. doi: 10.1039/D0QO01480J. [DOI] [Google Scholar]; d Acharya S. S., Rout P. R., Sutar R., Pradhan C., Parida B. B.. Transition Metal-Catalyzed Directing Group-Assisted Site-Selective Di-ortho C–H Functionalizations via Double C–H Activation. Adv. Synth. Catal. 2025:2500184. doi: 10.1002/adsc.202500184. [DOI] [Google Scholar]; e Singh B. D., Pandey J., Khanam H., Tiwari B., Azeez T., Mishra A., Kanchan P.. Copper(ii) nanodots stabilized on Cassia fistula galactomannan: preparation and catalytic application towards fast solvent-free Biginelli reactions. Org. Biomol. Chem. 2024;22:3955–3965. doi: 10.1039/D4OB00441H. [DOI] [PubMed] [Google Scholar]; f Sahoo K., Patra N., Dandela R., Thirupathi B.. A Three-Component Synthesis of Spiropyrrolines. J. Org. Chem. 2024;89:5337–5352. doi: 10.1021/acs.joc.3c02713. [DOI] [PubMed] [Google Scholar]; g Kumar R., Acharya S. S., Bhaumick P., Parvin T., Choudhury L. H.. HFIP-mediated multicomponent reactions for the synthesis of fluorescent quinoline-fused pyrroles. Tetrahedron. 2023;132:133250. doi: 10.1016/j.tet.2023.133250. [DOI] [Google Scholar]; h Acharya S. S., Samantaray D., Sibakrishna C., Parida B. B.. Pseudo-Multicomponent Reactions of Lawsone: Synthetic Strategies of Bis-Lawsone. ChemistrySelect. 2025;10:e202403416. doi: 10.1002/slct.202403416. [DOI] [Google Scholar]; i Kumari V., Acharya S. S., Mondal N., Choudhury L. H.. Maleimide-Dependent Rh(III)-Catalyzed Site-Selective Mono and Dual C–H Functionalization of 2-Arylbenzo[d]thiazole and Oxazole Derivatives. J. Org. Chem. 2024;89:18003–18018. doi: 10.1021/acs.joc.4c01615. [DOI] [PubMed] [Google Scholar]; j Kiyani H., Kanaani A., Ajloo D., Ghorbani F., Vakili M.. N-bromosuccinimide (NBS)-promoted, three-component synthesis of α,β-unsaturated isoxazol-5(4H)-ones, and spectroscopic investigation and computational study of 3-methyl-4-(thiophen-2-ylmethylene)isoxazol-5(4H)-one. Res. Chem. Intermed. 2015;41:7739–7773. doi: 10.1007/s11164-014-1857-5. [DOI] [Google Scholar]; k Delfani A. M., Kiyani H., Zamani M.. Synthesis of Tetrahydrobenzo[b]pyrans Catalyzed by 1,3-Dibenzyl-1H-benzo[d] imidazole-3-ium Chloride. Curr. Org. Chem. 2023;27:1542–1552. doi: 10.2174/0113852728269951231009060535. [DOI] [Google Scholar]
- a Toure B. B., Hall D. G.. Natural Product Synthesis Using Multicomponent Reaction Strategies. Chem. Rev. 2009;109:4439–4486. doi: 10.1021/cr800296p. [DOI] [PubMed] [Google Scholar]; b Larghi E. L., Bracca A. B. J., Simonetti S. O., Kaufman T. S.. Recent developments in the total synthesis of natural products using the Ugi multicomponent reactions as the key strategy. Org. Biomol. Chem. 2024;22:429–465. doi: 10.1039/D3OB01837G. [DOI] [PubMed] [Google Scholar]; c Ostadzadeh H., Kiyani H.. Multicomponent Synthesis of Tetrahydrobenzo[b]Pyrans, Pyrano[2,3-d]Pyrimidines, and Dihydropyrano[3,2-c]Chromenes Catalyzed by Sodium Benzoate. Polycycl. Aromat. Compd. 2023;43:9318–9337. doi: 10.1080/10406638.2022.2162091. [DOI] [Google Scholar]
- a Slobbe P., Ruijter E., Orru R. V. A.. Recent applications of multicomponent reactions in medicinal chemistry. Med. Chem. Commun. 2012;3:1189–1218. doi: 10.1039/c2md20089a. [DOI] [Google Scholar]; b Soni S., Teli S., Teli P., Manhas A., Jha P. C., Agarwal S.. Highly efficient synthesis of isoxazolones and pyrazolones using g-C3N4·OH nanocomposite with their in silico molecular docking, pharmacokinetics and simulation studies. Sci. Rep. 2024;14:19123. doi: 10.1038/s41598-024-70071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Lamberth C.. Multicomponent reactions in crop protection chemistry. Bioorg. Med. Chem. 2020;28:115471. doi: 10.1016/j.bmc.2020.115471. [DOI] [PubMed] [Google Scholar]; b Acharya S. S., Sahoo R. K., Mohanty P., Panda P., Parida B. B.. Arylglyoxal-based multicomponent synthesis of C-3 functionalized imidazoheterocycles. Chem. Pap. 2024;78:8107–8126. doi: 10.1007/s11696-024-03674-1. [DOI] [Google Scholar]
- Meier M. A. R., Hu R., Tang B. Z.. Multicomponent Reactions in Polymer Science. Macromol. Rapid Commun. 2021;42:2100104. doi: 10.1002/marc.202100104. [DOI] [PubMed] [Google Scholar]
- Reguera L., Mendez Y., Humpierre A. R., Valdes O., Rivera D. G.. Multicomponent Reactions in Ligation and Bioconjugation Chemistry. Acc. Chem. Res. 2018;51:1475–1486. doi: 10.1021/acs.accounts.8b00126. [DOI] [PubMed] [Google Scholar]
- a Zhang X., Wu G., Huo L., Guo X., Qiu S., Liu H., Tan H., Hu Y.. The First Racemic Total Syntheses of the Antiplasmodials Watsonianones A and B and Corymbone B. J. Nat. Prod. 2020;83:3–7. doi: 10.1021/acs.jnatprod.8b01077. [DOI] [PubMed] [Google Scholar]; b Sun C., Zhao W., Wang X., Sun Y., Chen X.. A pharmacological review of dicoumarol: An old natural anticoagulant agent. Pharmacol. Res. 2020;160:105193. doi: 10.1016/j.phrs.2020.105193. [DOI] [PubMed] [Google Scholar]
- Khan K. M., Maharvi G. M., Khan M. T. H., Jabbar Shaikh A., Perveen S., Begum S., Choudhary M. I.. Tetraketones: A new class of tyrosinase inhibitors. Bioorg. Med. Chem. 2006;14:344–351. doi: 10.1016/j.bmc.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Kali V., Maiti B.. The expediency of [BCMIM][Cl] ionic salt in the synthesis of tetraketones: Insights into their photophysical properties and theoretical calculations. J. Mol. Struct. 2024;1299:137053. doi: 10.1016/j.molstruc.2023.137053. [DOI] [Google Scholar]
- a Chaudhary H. R., Patel P. J., Gupta V. K., Patel D. M.. L-proline-Brønsted acid deep eutectic mixture (DEM) triggered consecutive Claisen–Schmidt and Michael addition reactions. Res. Chem. Intermed. 2024;50:1273–1286. doi: 10.1007/s11164-023-05215-z. [DOI] [Google Scholar]; b Azizi N., Dezfooli S., Hashemi M. M.. Chemoselective synthesis of xanthenes and tetraketones in a choline chloride-based deep eutectic solvent. C. R. Chim. 2013;16:997–1001. doi: 10.1016/j.crci.2013.05.002. [DOI] [Google Scholar]; c Abbasi F., Azizi N., Abdoli-Senejani M.. Highly efficient synthesis of dicoumarols and xanthene derivatives in presence of Brønsted–Lewis acidic ionic liquids catalyst. J. Iran. Chem. Soc. 2017;14:2097–2103. doi: 10.1007/s13738-017-1146-5. [DOI] [Google Scholar]; d El-Nassan H. B., El-Mosallamy S. S., Mahmoud A. M.. Sustain. Chem. Pharm. 2023;35:101207. doi: 10.1016/j.scp.2023.101207. [DOI] [Google Scholar]; e Mondal S., Pandey A. M., Gnanaprakasam B.. Continuous-flow Fe-zeolite-catalyzed temperature-directed synthesis of bioactive tetraketones and xanthenes using epoxides and cyclic-1,3-diketones via a Meinwald rearrangement. React. Chem. Eng. 2023;8:855–862. doi: 10.1039/d2re00452f. [DOI] [Google Scholar]; f Azizi N., Abbasi F., Abdoli-Senejani M.. Natural Acidic Ionic Liquid Immobilized on Magnetic Silica: Preparation and Catalytic Performance in Chemoselective Synthesis of Dicoumarols and Substituted Xanthene Derivatives. ChemistrySelect. 2018;3:3797–3802. doi: 10.1002/slct.201800138. [DOI] [Google Scholar]
- a Teli P., Sahiba N., Sethiya A., Soni J., Agarwal S.. Advancement in synthetic strategies of bisdimedones: Two decades study. J. Heterocycl. Chem. 2021;58:1393–1407. doi: 10.1002/jhet.4239. [DOI] [Google Scholar]; b Hasanzadeh Banakar S., Dekamin M. G., Yaghoubi A.. Selective and highly efficient synthesis of xanthenedione or tetraketone derivatives catalyzed by ZnO nanorod-decorated graphene oxide. New J. Chem. 2018;42:14246–14262. doi: 10.1039/c8nj01053f. [DOI] [Google Scholar]; c Patel M. S., Parekh J. N., Chudasama D. D., Patel H. C., Dalwadi P., Kunjadiya A., Bhatt V., Ram K. R.. Meglumine-Promoted Eco-Compatible Pseudo-Three-Component Reaction for the Synthesis of 1,1-Dihomoarylmethane Scaffolds and Their Green Credentials. ACS Omega. 2022;7:30420–30439. doi: 10.1021/acsomega.2c03787. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Dashteh M., Baghery S., Zolfigol M. A., Bayat Y., Asgari A.. 1,10-Phenanthrolin-1-ium Trinitromethanide (1,10-PHTNM) as a Nano Molten Salt Catalyst With Y-Aromatic Counter Ion: Applications for Synthesis of Organic Compounds. ChemistrySelect. 2019;4:337–346. doi: 10.1002/slct.201803402. [DOI] [Google Scholar]; e Kamalifar S., Kiyani H.. Facile and Efficient Synthesis of 9-Aryl-1,8-DioxoOctahydroxanthenes Catalyzed by Sulfacetamide. Polycycl. Aromat. Compd. 2022;42:3675–3693. doi: 10.1080/10406638.2021.1872656. [DOI] [Google Scholar]; f Soni S., Teli P., Sahiba N., Teli S., Agarwal S.. Exploring the synthetic potential of a g-C3N4·SO3H ionic liquid catalyst for one-pot synthesis of 1,1-dihomoarylmethane scaffolds via Knoevenagel–Michael reaction. RSC Adv. 2023;13:13337–13353. doi: 10.1039/D3RA01971C. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Teli P., Sethiya A., Agarwal S.. Black yet green: A heterogenous carbon-based acid catalyst for the synthesis of biscyclic derivatives under eco-friendly conditions. Res. Chem. Intermed. 2022;48:731–750. doi: 10.1007/s11164-021-04622-4. [DOI] [Google Scholar]
- Borah B., Swain S., Patat M., Kumar B., Prajapat K. K., Biswas R., Vasantha R., Chowhan L. R.. Brønsted acid catalyzed mechanochemical domino multicomponent reactions by employing liquid assisted grindstone chemistry. Sci. Rep. 2023;13:1386. doi: 10.1038/s41598-023-27948-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta P., Singh B., Dhama M., Pani B., Mozumdar S.. A biscationic imidazolium ionic liquid immobilized on graphene oxide as an efficient heterogeneous catalyst for the synthesis of tetraketone derivatives. New J. Chem. 2024;48:1518–1527. doi: 10.1039/D3NJ03812B. [DOI] [Google Scholar]
- Mondal S., Pandey A. M., Gnanaprakasam B.. Visible Light Mediated Organophotoredox Catalyzed Synthesis of Tetraketones Using Tertiary Amines as Alkyl Synthons. J. Org. Chem. 2024;89:3769–3780. doi: 10.1021/acs.joc.3c02613. [DOI] [PubMed] [Google Scholar]
- a Patra S. S., Panda S., Acharya S. S., Phaomei G., Parida B. B.. Green and Sustainable Synthesis of Biaryls Using LaPO4·Pd Recyclable Nanocatalyst by the Suzuki–Miyaura Coupling in Aqueous Medium. ACS Omega. 2025;10:24105. doi: 10.1021/acsomega.4c10467. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Acharya S. S., Guin B., Parida B. B.. One-Pot Multicomponent Synthesis of Fully Substituted 1,3-Thiazoles Appended with Naturally Occurring Lawsone. J. Org. Chem. 2025;90:2717–2727. doi: 10.1021/acs.joc.4c02927. [DOI] [PubMed] [Google Scholar]; c Panda S., Patra S., Acharya S. S., Phaomei G., Parida B. B.. Recyclable LaF3·Pd nanocatalyst in Suzuki coupling: green synthesis of biaryls from haloarenes and phenylboronic acids. RSC Adv. 2024;14:21269–21276. doi: 10.1039/D4RA00686K. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Choudhury S. K., Rout P., Parida B. B., Florent J.-C., Johannes L., Phaomei G., Bertounesque E., Rout L.. Metal-Free Activation of C(sp3)–H Bond, and a Practical and Rapid Synthesis of Privileged 1-Substituted 1,2,3,4-Tetrahydroisoquinolines. Eur. J. Org Chem. 2017;2017:5275–5292. doi: 10.1002/ejoc.201700471. [DOI] [Google Scholar]; e Rout L., Parida B. B., Florent J.-C., Johannes L., Choudhury S. K., Phaomei G., Scanlon J., Bertounesque E.. Metal-Free Activation of a C(sp)–H Bond of Aryl Acetylenes. Chem.Eur. J. 2016;22:14812–14815. doi: 10.1002/chem.201603003. [DOI] [PubMed] [Google Scholar]; f Acharya S. S., Rout P. R., Barad L. M., Dansana J., Gadtya A., Meher B. R., Parida B. B.. Visible light-driven photocatalyst-free cascade synthesis of functionalized benzopyrans as inhibitors of Chikungunya nsPs and Zika NS2B-NS3. Org. Biomol. Chem. 2025;23:8465–8473. doi: 10.1039/D5OB01283J. [DOI] [PubMed] [Google Scholar]; g Acharya S. S., Barad L. M., Rout P. R., Bisoyi A., Parida B. B.. Ultrasonication-assisted Multicomponent Green and Sustainable Synthesis of Benzopyrans Employing Taurine as a Bioorganic catalyst. New J. Chem. 2025;49:12090–12101. doi: 10.1039/D4NJ05110F. [DOI] [Google Scholar]
- a Tiwari J., Saquib M., Singh S., Tufail F., Singh M., Singh J., Singh J.. Visible light promoted synthesis of dihydropyrano[2,3-c]chromenes via a multicomponent-tandem strategy under solvent and catalyst free conditions. Green Chem. 2016;18:3221–3231. doi: 10.1039/c5gc02855h. [DOI] [Google Scholar]; b Kumari, S. ; Maury, S. K. ; Singh, H. K. ; Kamal, A. ; Kumar, D. ; Singh, S. ; Srivastava, V. . Visible Light Mediated, Photocatalyst-Free Condensation of Barbituric Acid with Carbonyl Compounds. ChemistrySelect, 6, 2980–2987. 10.1002/slct.202100051 [DOI] [Google Scholar]; c Ghosh P. P., Paul S., Das A. R.. Light induced synthesis of symmetrical and unsymmetrical dihydropyridines in ethyl lactate–water under tunable conditions. Tetrahedron Lett. 2013;54:138–142. doi: 10.1016/j.tetlet.2012.10.106. [DOI] [Google Scholar]; d Ghosh S., Saikh F., Das J., Pramanik A. K.. Hantzsch 1,4-dihydropyridine synthesis in aqueous ethanol by visible light. Tetrahedron Lett. 2013;54:58–62. doi: 10.1016/j.tetlet.2012.10.079. [DOI] [Google Scholar]; e Nazeef M., Shivhare K. N., Ali S., Ansari S., Siddiqui I. R.. Visible-light-mediated one-pot efficient synthesis of 1-aryl-1H,3H-thiazolo[3,4-a]benzimidazoles: a metal-free photochemical approach in aqueous ethanol. Mol. Divers. 2021;25:2479–2486. doi: 10.1007/s11030-020-10145-8. [DOI] [PubMed] [Google Scholar]; f Nadaf A. N., Shivashankar K.. Visible Light-Induced Synthesis of Biscoumarin Analogs under Catalyst-Free Conditions. J. Heterocycl. Chem. 2018;55:1375–1381. doi: 10.1002/jhet.3171. [DOI] [Google Scholar]; g Gupta A., Iqbal S., Roohi, Hussain M. K., Zaheer M. R., Shankar K.. Visible Light-Promoted Green and Sustainable Approach for One-Pot Synthesis of 4,4’-(Arylmethylene)bis(1H-pyrazol-5-ols), In Vitro Anticancer Activity, and Molecular Docking with Covid-19 Mpro . ACS Omega. 2022;7:34583–34598. doi: 10.1021/acsomega.2c04506. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Hoffmann N.. Photochemical Reactions as Key Steps in Organic Synthesis. Chem. Rev. 2008;108:1052–1103. doi: 10.1021/cr0680336. [DOI] [PubMed] [Google Scholar]
- Rajmane A., Patil N., Patil A., Kamble S., Kumbhar A.. DABCO dicationic ionic solid supported polymer (DDIS@PS) mediated synthesis of diverse 2-amino-4H-chromenes and xanthenes: a cascade Knoevenagel–Michael approach. New J. Chem. 2025;49:3644. doi: 10.1039/D4NJ04366A. [DOI] [Google Scholar]
- Gilanizadeh M., Zeynizadeh B.. Synthesis and characterization of the immobilized Ni–Zn–Fe layered double hydroxide (LDH) on silica-coated magnetite as a mesoporous and magnetically reusable catalyst for the preparation of benzylidenemalononitriles and bisdimedones (tetraketones) under green conditions. New J. Chem. 2018;42:8553–8566. doi: 10.1039/c8nj00788h. [DOI] [Google Scholar]
- Bhardwaj M., Sahi S., Mahajan H., Paul S., Clark J. H.. Novel heterogeneous catalyst systems based on Pd(0) nanoparticles onto amine functionalized silica-cellulose substrates [Pd(0)-EDA/SCs]: Synthesis, characterization and catalytic activity toward C–C and C–S coupling reactions in water under limiting basic conditions. J. Mol. Catal. A:Chem. 2015;408:48–59. doi: 10.1016/j.molcata.2015.07.005. [DOI] [Google Scholar]
- Ilangovan A., Malayappasamy S., Muralidharan S., Maruthamuthu S.. A highly efficient green synthesis of 1, 8-dioxo-octahydroxanthenes. Chem. Cent. J. 2011;5:81. doi: 10.1186/1752-153x-5-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kour M., Paul S.. Sulfonated carbon/nano-metal oxide composites: a novel and recyclable solid acid catalyst for organic synthesis in benign reaction media. New J. Chem. 2015;39:6338–6350. doi: 10.1039/C5NJ00607D. [DOI] [Google Scholar]
- Gharghish S., Dekamin M. G., Banakar S. H.. Functionalized graphene oxide by 4-amino-3-hydroxy-1-naphthalenesulfonic acid as a heterogeneous nanocatalyst for the one-pot synthesis of tetraketone and tetrahydrobenzo[b]pyran derivatives under green conditions. Nanoscale Adv. 2024;6:3911–3922. doi: 10.1039/D4NA00223G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanzadeh Banakar S., Dekamin M. G., Yaghoubi A.. Selective and highly efficient synthesis of xanthenedione or tetraketone derivatives catalyzed by ZnO nanorod-decorated graphene oxide. New J. Chem. 2018;42:14246–14262. doi: 10.1039/c8nj01053f. [DOI] [Google Scholar]
- Lasemi Z., Tajbakhsh M., Alinezhad H., Mehrparvar F.. 1,8-Diazabicyclo [5.4.0] undec-7-ene functionalized cellulose nanofibers as an efficient and reusable nanocatalyst for the synthesis of tetraketones in aqueous medium. Res. Chem. Intermed. 2020;46:3667–3682. doi: 10.1007/s11164-020-04167-y. [DOI] [Google Scholar]
- Sapkal B. M., Labhane P. K., Satam J. R.. In water–ultrasound-promoted synthesis of tetraketones and 2-substituted-1H-benzimidazoles catalyzed by BiOCl nanoparticles. Res. Chem. Intermed. 2017;43:4967–4979. doi: 10.1007/s11164-017-2924-5. [DOI] [Google Scholar]
- Safaei-Ghomi J., Asadian S., Nazemzadeh S. H., Shahbazi-Alavi H.. Synthesis of Tetraketones Using ZnS Nanoparticles as an Efficient Catalyst. J. Chin. Chem. Soc. 2018;65:430–434. doi: 10.1002/jccs.201700250. [DOI] [Google Scholar]
- Rahmani S., Zeynizadeh B.. Ni0 NPs anchored on acid-activated montmorillonite (Ni0-Mont) as a highly efficient and reusable nanocatalyst for synthesis of biscoumarins and bisdimedones. Res. Chem. Intermed. 2019;45:1227–1248. doi: 10.1007/s11164-018-3671-y. [DOI] [Google Scholar]
- Vajar S., Mokhatry M.. Nano-CuFe2O4@SO3H Catalyzed Efficient One-Pot Cyclo-Dehydration of Dimedone and Synthesis of Chromeno[4,3-b]chromenes. Polycyclic Aromat. Compd. 2019;39:111–123. doi: 10.1080/10406638.2017.1280516. [DOI] [Google Scholar]
- Tajbakhsh M., Heidary M., Hosseinzadeh R.. Nano Fe/NaY zeolite: an efficient and reusable solid-supported catalyst for synthesis of 1-oxo-hexahydroxanthene and tetraketone derivatives. Res. Chem. Intermed. 2016;42:1425–1439. doi: 10.1007/s11164-015-2094-2. [DOI] [Google Scholar]
- Kidwai M., Bansal V., Mothsra P., Saxena S., Somvanshi R. K., Dey S., Singh T. P.. Molecular iodine: A versatile catalyst for the synthesis of bis(4-hydroxycoumarin) methanes in water. J. Mol. Catal. A:Chem. 2007;268:76–81. doi: 10.1016/j.molcata.2006.11.054. [DOI] [Google Scholar]
- Goodsell D. S., Olson A. J.. Automated docking of substrates to proteins by simulated annealing. Proteins. 1990;8:195–202. doi: 10.1002/prot.340080302. [DOI] [PubMed] [Google Scholar]
- Miller B. R., McGee T. D., Swails J. M., Homeyer N., Gohlke H., Roitberg A. E.. MMPBSA.py: An Efficient Program for End-State Free Energy Calculations. J. Chem. Theory Comput. 2012;8:3314–3321. doi: 10.1021/ct300418h. [DOI] [PubMed] [Google Scholar]
- Krieger E., Nielsen J. E., Spronk C. A. E. M., Vriend G.. Fast empirical pKa prediction by Ewald summation. J. Mol. Graph. Model. 2006;25:481–486. doi: 10.1016/j.jmgm.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Meher B. R., Wang Y.. Interaction of I50V Mutant and I50L/A71V Double Mutant HIV-Protease with Inhibitor TMC114 (Darunavir): Molecular Dynamics Simulation and Binding Free Energy Studies. J. Phys. Chem. B. 2012;116:1884–1900. doi: 10.1021/jp2074804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meher B. R., Wang Y.. Binding of single-walled carbon nanotube to WT and mutant HIV-1 proteases: Analysis of flap dynamics and binding mechanism. J. Mol. Graph. Model. 2012;38:430–445. doi: 10.1016/j.jmgm.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meher B. R., Wang Y.. Exploring the drug resistance of V32I and M46L mutant HIV-1 protease to inhibitor TMC114: Flap dynamics and binding mechanism. J. Mol. Graph. Model. 2015;56:60–73. doi: 10.1016/j.jmgm.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panda M., Purohit P., Wang Y., Meher B. R.. Functionalized carbon nanotubes as an alternative to traditional anti-HIV-1 protease inhibitors: An understanding towards Nano-medicine development through MD simulations. J. Mol. Graph. Model. 2022;117:108280. doi: 10.1016/j.jmgm.2022.108280. [DOI] [PubMed] [Google Scholar]
- Purohit P., Dash J. J., Muya J. T., Meher B. R.. Molecular insights to the binding interactions of APNS containing HIV-protease inhibitors against SARS-CoV-2 Mpro: an in silico approach towards drug repurposing. J. Biomol. Struct. Dyn. 2023;41:3900–3913. doi: 10.1080/07391102.2022.2059008. [DOI] [PubMed] [Google Scholar]
- Purohit P., Sahoo S., Panda M., Sahoo P. S., Meher B. R.. Targeting the DENV NS2B-NS3 protease with active antiviral phytocompounds: structure-based virtual screening, molecular docking and molecular dynamics simulation studies. J. Mol. Model. 2022;28:365. doi: 10.1007/s00894-022-05355-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo S., Purohit P., Samantaray S., Meher B. R.. Identification of Antiviral Phytocompounds as Potential Anti-Dengue Agents against DENV NS2B/NS3 Protease: An Integrated Molecular Modelling and Dynamics Approach. ChemistrySelect. 2024;9:e202400384. doi: 10.1002/slct.202400384. [DOI] [Google Scholar]
- Purohit P., Barik D., Agasti S., Panda M., Meher B. R.. Evaluation of the inhibitory potency of anti-dengue phytocompounds against DENV-2 NS2B-NS3 protease: virtual screening, ADMET profiling and molecular dynamics simulation investigations. J. Biomol. Struct. Dyn. 2024;42:2990–3009. doi: 10.1080/07391102.2023.2212798. [DOI] [PubMed] [Google Scholar]
- Purohit P., Barik D., Dansana J., Meher B. R.. Investigating Lycotoxin-An1a (An1a), a defense antiviral peptide from Alopecosa nagpag venom as prospective anti-dengue agent against DENV-2 NS2B-NS3 protease. Comput. Biol. Chem. 2024;108:108005. doi: 10.1016/j.compbiolchem.2023.108005. [DOI] [PubMed] [Google Scholar]
- Weinhold F., Landis C. R., Glendening E. D.. What is NBO analysis and how is it useful? Int. Rev. Phys. Chem. 2016;35:399–440. doi: 10.1080/0144235X.2016.1192262. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.