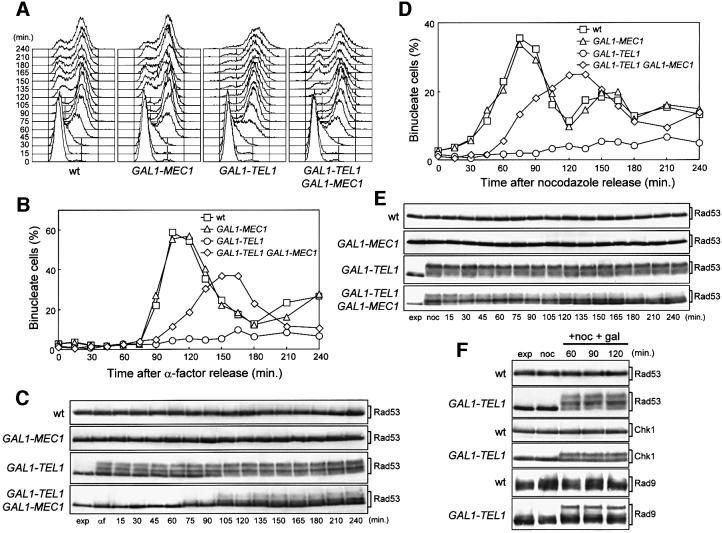
Fig. 1. Effects of TEL1 overexpression during an unperturbed cell cycle. Strains were as follows: wild type (K699), GAL1–MEC1 (YLL516), GAL1–TEL1 (DMP3539/10D) and GAL1–TEL1 GAL1–MEC1 (DMP3539/9D). (A–C) Cell cultures, growing logarithmically in YEP-raf, were synchronized in G1 with α-factor in the presence of galactose (2 h). Release from α-factor block was performed at time zero (C, αf) by transferring cell cultures to fresh YEP-raf-gal medium. Samples were taken at the times indicated after release into the cell cycle to determine the DNA content by fluorescence-activated cell sorter (FACS) analysis (A), the kinetics of nuclear division by direct visualization by propidium iodide staining (B) and the amount and phosphorylation of Rad53 by western blotting of protein extracts with anti-Rad53 antibodies (C). (D and E) Cell cultures, growing logarithmically in YEP-raf, were synchronized in G2 with nocodazole in the presence of galactose (2 h). Release from nocodazole block was performed at time zero (E, noc) by transferring cell cultures to fresh YEP-raf-gal medium. Samples were taken at the times indicated after release from nocodazole to determine the kinetics of nuclear division (D) and Rad53 levels and phosphorylation (E) as described in (B) and (C), respectively. (F) Cell cultures of wild-type (DMP3598/21D) and GAL1–TEL1 (DMP3598/6A) strains were arrested with nocodazole in YEP-raf medium (noc) and resuspended in YEP-raf-gal medium containing 15 µg/ml nocodazole (+noc +gal). Protein extracts were prepared from samples withdrawn at the times indicated and analyzed by western blotting. The slowly migrating protein species specifically reacting with anti-Rad53, anti-MYC (Chk1) and anti-Rad9 antibodies represent phosphorylated forms of the corresponding proteins (Sanchez et al., 1996; Vialard et al., 1998, 1999). exp, exponentially growing cells.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.