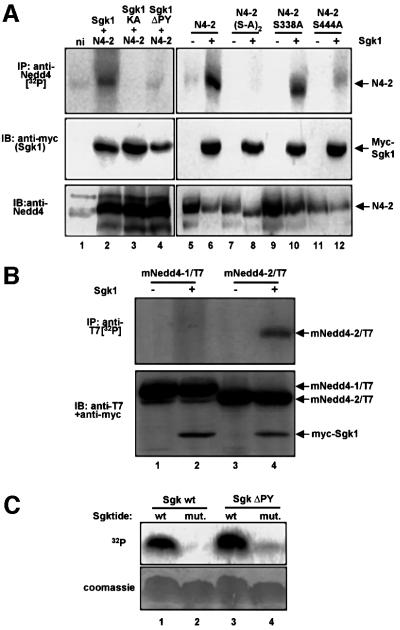
Fig. 2. Sgk1 phosphorylates Nedd4-2, but not Nedd4-1. (A) Oocytes expressing either wild-type Nedd4-2 (N4-2) or the phosphorylation mutants Nedd4-2-S338A-S444A [N4-2(S-A)2], Nedd4-2-S338A, Nedd4-2-S444A and myc-Sgk1 [wild-type, catalytically inactive (KA, K130A) or PY motif-mutated (ΔPY, Y301A)] were incubated with [32P]ortho–phosphate and treated as follows: top, immunoprecipitation from lysates with anti-Nedd4-2 antibodies and autoradiography; middle, western blot on lysates with anti-myc antibody (recognizing Sgk1); bottom, western blot on lysates with anti-Nedd4-2 antibodies. (B) Mouse Nedd4-1 (mNedd4-1/T7) or Nedd4-2 (mNedd4-2/T7), both epitope-tagged with a T7 epitope (Novagen), were expressed with or without Sgk1 (as indicated) and phosphorylation was followed as described in (A), except that the Nedd4 proteins were immunoprecipitated with anti-T7 antibody (top). Expression of mNedd4-1, mNedd4-2 and Sgk1 was followed by western blot analysis on lysates, using both anti-T7 (Nedd4-1 or Nedd4-2) and anti-myc (Sgk1) antibodies. (C) Phosphorylation of a synthetic peptide substrate (Sgktide) by Sgk1. Wild-type and mutant myc-Sgk1 (lacking a functional PY motif) were expressed in Xenopus oocytes and immunoprecipitated with anti-myc antibodies. The immunoprecipitated kinases were assayed in a kinase assay with Sgktide or a mutant peptide lacking the phosphorylation site as described in Materials and methods. Phosphorylated peptides were then analyzed by separation on a tricine acrylamide gel followed by autoradiography. Both wild-type and mutant Sgk1 were able to phosphorylate Sgktide, but not its mutant (top). Bottom, Coomassie Blue staining.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.