Abstract
Background
The global prevalence of obesity and metabolic disorders has reached critical levels, with over 1 billion individuals affected as of 2024. Traditional dietary strategies focusing on caloric restriction and macronutrient composition have yielded modest success. Emerging evidence suggests that when food is consumed, termed circadian nutrition, is an influential, yet underutilized factor in metabolic regulation.
Objective
This narrative review examines how aligning meal timing with endogenous circadian rhythms modulates energy balance, hormonal regulation, and adiposity. It integrates recent mechanistic insights and synthesizes evidence from both animal and human studies to explore the metabolic impact of circadian-aligned eating patterns.
Methods
A comprehensive review of peer-reviewed literature (2013–2025) was conducted using PubMed, Scopus, and ScienceDirect, focusing on studies of circadian rhythms, nutrient timing, time-restricted eating (TRE), and metabolic outcomes. English-language human trials and mechanistic animal studies, relevant systematic reviews/meta-analyses were consulted. Key findings were synthesized across clinical trials, observational cohorts, and experimental models.
Key findings
Meal timing exerts significant effects on glucose metabolism, lipid regulation, and inflammatory pathways. Importantly, emerging evidence from animal models with isocaloric controls suggests that these benefits are not solely due to reduced caloric intake but also reflect independent effects of aligning food intake with circadian rhythms. Consuming a higher proportion of energy earlier in the day, with potentially more favorable distributions of carbohydrates, protein, and micronutrients, avoiding late-night eating, and practicing time-restricted feeding have been associated with improvements in insulin sensitivity, weight regulation, and cardiometabolic health. Disruptions in circadian rhythms, as seen in shift workers or individuals with irregular eating schedules, contribute to metabolic dysregulation and obesity risk.
Conclusion
Circadian-aligned eating may offer a feasible adjunct to standard dietary strategies, but effect sizes remain uncertain given that much of the literature comprises small, short-term, heterogeneous trials. Larger, longer, and more diverse RCTs and pragmatic studies are needed to establish durability, clinical significance, and population-specific guidance.
Graphical abstract
Keywords: Circadian rhythms, Meal timing, Obesity, Metabolic health, Time-restricted eating, Chrononutrition, Nutrient timing, Insulin sensitivity, Adiposity, Hormonal regulation
1. Introduction
The global burden of obesity continues to escalate, posing a significant challenge to public health systems worldwide, contributing to rising incidences of type 2 diabetes, cardiovascular diseases, and certain cancers [1]. Obesity, defined as abnormal or excessive fat accumulation that impairs health, is clinically classified using the body mass index (BMI), with a threshold of ≥ 30 kg/m² in adults and age- and sex-specific percentile cutoffs in children and adolescents. According to the World Health Organization (WHO), obesity has nearly tripled since 1975, with over 650 million adults affected as of 2016. In 2022, obesity affected 16% of adults worldwide, affecting 890 million individuals. In 2024, the global population of individuals with obesity exceeded 1 billion, including 159 million children and adolescents [2]. Despite decades of nutritional research and public health interventions, the trajectory remains upward, underscoring the need for innovative and multidimensional approaches to prevention and management. Traditional nutritional strategies for obesity management have predominantly emphasized caloric restriction, macronutrient composition, and dietary quality [3]. While these components remain critical, emerging evidence points to a novel and an influential determinant of metabolic health called meal timing. This concept, known as circadian nutrition or chrononutrition, posits that aligning food intake with the body’s endogenous circadian rhythms can significantly influence energy homeostasis, hormonal signaling, and adiposity regulation [4].
The human body is governed by endogenous circadian rhythms, which is approximately 24-hour cycles that regulate various physiological processes, including hormone secretion, glucose metabolism, and energy expenditure. These rhythms are orchestrated by a central pacemaker in the suprachiasmatic nucleus (SCN) of the hypothalamus and are influenced by external cues such as light exposure and feeding cycles [5]. Peripheral clocks in organs such as the liver, pancreas, adipose tissue, and gastrointestinal tract interact with the central clock to maintain metabolic homeostasis [6]. When feeding behavior is misaligned with these circadian signals, as observed in shift workers, individuals with irregular eating schedules, or those who engage in late-night eating, there is a heightened risk of weight gain, insulin resistance, and cardiometabolic dysfunction [7, 8].
While caloric content and macronutrient composition remain foundational, accumulating research demonstrates that when we eat is equally critical. Time-restricted eating (TRE), early time-restricted feeding (eTRE), and other temporal strategies have shown promising effects on glycemic control, blood pressure, inflammatory markers, and body weight even in the absence of calorie reduction. This emerging paradigm challenges the long-standing notion that “a calorie is a calorie,” highlighting the need to integrate temporal patterns into dietary guidelines and interventions [9–11]. Unlike traditional dietary interventions that focus solely on the quantity and quality of food, circadian nutrition introduces the dimension of temporal alignment, which may provide a low-cost, behaviorally feasible, and metabolically useful approach to obesity prevention and treatment [12, 13]. Despite the compelling evidence, there is limited long-term, population-specific, and guideline-oriented evidence for circadian nutrition.
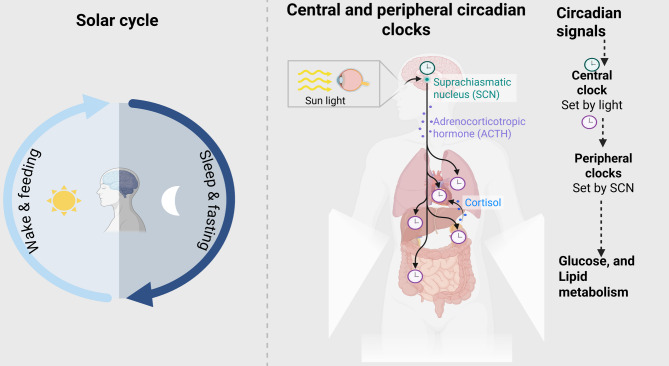
This review synthesizes current mechanistic and clinical evidence on circadian nutrition and obesity, emphasizing its potential as a low-cost, behaviorally feasible, and physiologically grounded approach to metabolic health. Key hormonal mediators such as cortisol and adrenocorticotropic hormone (ACTH), which follow pronounced circadian rhythms, also play integral roles in linking central clock signals to metabolic regulation and are highlighted in Fig. 1. It also outlines critical knowledge gaps and future directions, including the need for personalized chrononutrition models that incorporate chronotype, sociocultural norms, and long-term adherence potential. Ultimately, this narrative aims to reposition meal timing as a core component of both clinical nutrition practice and public health policy in the fight against metabolic disease.
Fig. 1.
Diagram of the circadian system and peripheral metabolic regulation. The central clock in the suprachiasmatic nucleus (SCN) receives cues from the light-dark cycle and transmits circadian signals to peripheral clocks in organs such as the liver and adipose tissue, which regulate glucose and lipid metabolism. While the core molecular clock feedback loop involving CLOCK, BMAL1, PER, and CRY is central to circadian regulation, this is not depicted in Fig. 1, which instead focuses on system-level connections between the SCN, peripheral organs, and hormonal mediators
2. Methodology
This narrative review was conducted following best practices for non-systematic literature synthesis.
Databases searched/Time frame
Relevant peer-reviewed articles published between January 2013 and June 2025 were retrieved from PubMed, Scopus, and ScienceDirect. The literature search was restricted to 2013–2025 because chrononutrition research gained significant momentum after 2013, with most high-quality mechanistic and clinical studies published during this period.
Search terms
Search terms included combinations of “circadian nutrition,” “meal timing,” “time-restricted eating,” “chrononutrition,” “obesity,” “social jet lag”, and “metabolic health”.
Inclusion and exclusion criteria
Studies involving human participants or animal models and published in English were included especially those focusing on metabolic outcomes associated with temporal eating patterns. For animal studies, we focused on mammalian models (e.g., rodents, primates) given their circadian systems and metabolic regulation are most comparable to humans, and excluded invertebrate or non-mammalian studies that lack translational relevance.
Data synthesis and presentation
Data were synthesized using a narrative thematic framework, organized around biological mechanisms, clinical outcomes, and public health relevance. Emphasis was placed on high-quality randomized controlled trials (RCTs), longitudinal cohort studies, and translational insights from experimental models. The results were presented and discussed following the outlined thematic frameworks.
3. Circadian biology and metabolic regulation
The coordination of biological functions within the human body is largely governed by an internal timekeeping system known as the circadian clock. This system orchestrates physiological rhythms over a 24-hour period, regulating processes such as hormone secretion, metabolism, thermogenesis, and sleep-wake cycles [14]. Disruption of this tightly controlled rhythmicity, either through lifestyle behaviors (e.g., irregular eating, shift work) or environmental factors (e.g., light pollution), has been strongly associated with metabolic dysfunction and increased obesity risk [15]. To avoid conflation of related but distinct concepts, the following definitions are provided:
Meal timing
Refers to the distribution of eating occasions across the day (e.g., breakfast, lunch, dinner, and snacking), independent of fasting regimens.
Time-restricted eating (TRE)
Restricts caloric intake to a consistent daily window (6–10 h), aligning with the body’s active phase, without necessarily reducing overall calories.
Intermittent Fasting (IF): Involves alternating fasting and feeding periods across days or weeks (e.g., alternate-day fasting, 5:2 diet). IF primarily relies on caloric restriction or metabolic switching and is less circadian-focused than TRE.
Chrononutrition
An integrative framework studying the interaction between circadian rhythms, metabolic processes, and nutrition. Encompasses TRE, IF, and meal timing.
Social jet lag
Misalignment between biological circadian rhythms and social schedules (e.g., delayed eating due to work or social obligations), associated with increased metabolic risk.
3.1. The molecular clock system
At the core of circadian regulation lies a transcriptional–translational feedback loop composed of clock genes, including CLOCK, BMAL1, PER1-3, and CRY1-2. In the central pacemaker located in the suprachiasmatic nucleus (SCN) of the hypothalamus, the CLOCK and BMAL1 proteins dimerize and initiate the transcription of PER and CRY, whose protein products then inhibit CLOCK: BMAL1 activity, creating a ~ 24-hour rhythm of gene expression [16]. These oscillations are transmitted to peripheral clocks located in nearly all metabolically active tissues, including the liver, pancreas, skeletal muscle, adipose tissue, and gastrointestinal tract (Fig. 1). These peripheral oscillators respond not only to cues from the SCN but also to non-photic zeitgebers such as feeding-fasting cycles. Inappropriate feeding times (e.g., late-night eating) can uncouple peripheral clocks from the SCN, impairing processes such as glucose uptake, insulin secretion, and lipid oxidation [17]. In addition to these molecular mechanisms, key hormonal mediators such as cortisol and adrenocorticotropic hormone (ACTH) exhibit strong circadian rhythms that bridge central clock signals with peripheral metabolic regulation. Together, these pathways illustrate the integration of genetic, endocrine, and behavioral rhythms in maintaining energy homeostasis (Fig. 1). Disruption of circadian rhythms, governed by genes such as CLOCK, BMAL1, PER, and CRY, has been associated with negative metabolic outcomes, including obesity [18]. Studies indicate that obesity modifies the gene expression profile in peripheral blood mononuclear cells, with individuals with obesity demonstrating elevated levels of BMAL1, CRY1, CRY2, and PER2 relative to persons without obesity [19, 20]. Additionally, DNA methylation patterns of circadian genes have been linked to obesity, metabolic syndrome traits, and weight loss results [21]. The methylation status of particular CpG sites in the CLOCK and PER2 genes is associated with monounsaturated fat consumption and may serve as indicators for successful weight loss [21]. These findings highlight the intricate relationship among circadian rhythms, metabolism, and obesity.
3.2 Chronotype, hormonal Rhythms, and metabolic outcomes
Circadian regulation of metabolism is mediated by diurnal hormonal patterns, including insulin, leptin, ghrelin, cortisol, and melatonin. Insulin sensitivity peaks in the morning and declines across the day, favoring earlier caloric intake for optimal glucose handling. Leptin, secreted predominantly at night, signals satiety, while ghrelin surges before meals to stimulate hunger. Cortisol peaks early in the morning, supporting energy mobilization, whereas melatonin rises in the evening, impairing glucose tolerance and insulin secretion.
Importantly, individual chronotype, the biological predisposition toward morningness or eveningness, modifies these hormonal rhythms and their metabolic consequences. Morning chronotypes align better with diurnal insulin sensitivity and leptin signaling, whereas evening chronotypes are more likely to eat during melatonin secretion, leading to impaired glucose tolerance and weight gain. Recent evidence shows that carriers of the MTNR1B risk allele are particularly vulnerable to late eating, as melatonin-mediated suppression of insulin is amplified.
Together, hormonal rhythms and chronotype explain why seemingly contradictory findings emerge when examining meal timing. Rather than opposing one another, they highlight the interplay between universal biological rhythms and individual variability, underscoring the need for personalized chrononutrition strategies.
3.3. Synchronization with environmental cues
The circadian system requires regular external cues to maintain synchronization with the environment. Light is the dominant entrainer of the SCN, while feeding time is the primary synchronizer of peripheral clocks. When eating occurs during the rest phase (i.e., the biological night), metabolic processes that are typically inactive may be inappropriately activated, contributing to energy imbalance and fat storage [22]. However, in nocturnal animals, restricting food intake to the active phase (dark period) restores circadian alignment and protects against obesity, even in the presence of a high-fat diet [8]. Similarly, in humans, misalignment between eating time and internal circadian phase, as seen in night-shift workers results in disrupted glucose metabolism, reduced energy expenditure, and higher postprandial insulin and glucose levels [23].
3.4. Hormonal and metabolic outputs
Circadian rhythms tightly regulate key metabolic hormones, many of which follow diurnal patterns. For instance, insulin sensitivity peaks in the morning and declines in the evening, which has implications for glucose tolerance and diabetes risk [24]. Similar, leptin, a satiety hormone, shows nocturnal elevation, whereas ghrelin, the hunger hormone, peaks before meals and is influenced by meal regularity [25]. Additionally, cortisol, a glucocorticoid involved in glucose regulation and lipolysis, exhibits a sharp early morning peak and declines throughout the day [26]. This morning cortisol surge enhances gluconeogenesis and mobilizes energy substrates to support waking activity, meaning that food intake during this period may be metabolized more efficiently. In contrast, eating late in the evening, when cortisol and insulin sensitivity are low, favors nutrient storage and adiposity. Melatonin, secreted during the night, interacts with insulin secretion and glucose tolerance, often impairing postprandial glucose handling when meals are consumed during its peak [27]. A randomized, cross-over trial conducted by Lopez-Minguez and colleagues indicates that consuming dinner late at night adversely affects glucose tolerance and correlates with an elevated risk of type 2 diabetes. The effect was more pronounced in melatonin receptor MTNR1B risk-carriers compared to non-carriers [28]. The temporal orchestration of these hormones enables efficient energy utilization and storage in accordance with daily activity cycles. However, eating against the biological clock blunts these hormonal patterns, resulting in metabolic inflexibility, oxidative stress, and fat accumulation [29].
4. Evidence linking meal timing to obesity
A growing body of epidemiological and interventional research indicates that meal timing significantly influences energy balance, glucose metabolism, and body composition. Beyond total caloric intake and macronutrient distribution, when individuals eat plays a pivotal role in the development or prevention of obesity and its metabolic sequelae. In this section, we present evidence from human and animal studies that demonstrate the metabolic consequences of eating at biologically inappropriate times, including breakfast skipping, late-night eating, and chronically delayed feeding patterns.
4.1. Skipping breakfast and obesity risk
Numerous observational studies have consistently linked breakfast skipping with an increased risk of obesity and metabolic syndrome. In a longitudinal population-based with 27-year follow-up cohort study, breakfast skippers were found to have a significantly higher body mass index (BMI), increased waist circumference, and poorer glycaemic control compared to those who ate breakfast regularly [30]. Mechanistically, skipping breakfast may impair early-day insulin sensitivity, promote overeating later in the day, and disrupt circadian alignment of metabolism [31]. Controlled trials have shown that that front-loading (the practice of consuming a larger proportion of daily calories earlier in the day, especially at breakfast) is associated with greater weight loss, improved satiety, and better glycemic profiles [32]. The apparent paradox between observational studies linking breakfast skipping to obesity and intervention studies showing benefits of TRE, which often involves omitting breakfast, may be explained by context. Habitual breakfast skipping in free-living populations is frequently associated with irregular eating patterns, late-night energy intake, and poor diet quality, all of which disrupt circadian alignment and promote obesity. In contrast, structured TRE protocols establish a consistent, consolidated eating window, often aligned with the active phase of the day, thereby reducing circadian misalignment [33, 34]. Thus, it is not the mere absence of breakfast that drives outcomes, but rather the broader pattern of temporal alignment, regularity, and metabolic context. A meta-analysis of 45 observational studies revealed that individuals who omitted breakfast exhibited a 48% increased likelihood of being overweight or obese compared to those who regularly consumed breakfast [35]. Similarly, a meta-analysis of 44 trials reported that skipping breakfast was linked to heightened risks of diabetes, hypertension, and dyslipidaemia [36]. Conversely, a systematic review of longitudinal studies found only limited evidence connecting breakfast omission to weight gain, with an 11% increased risk of overweight/obesity when breakfast was skipped three or more days per week [37]. However, observational associations are hypothesis-generating rather than causal. Recent RCTs indicate outcomes are driven more by the eating window and circadian alignment than by the mere presence/absence of breakfast. Front-loading energy earlier in the day can improve appetite regulation and 24-h glycemia in some contexts, but effects are heterogeneous across populations and study designs. Late-evening intake continues to show experimentally poorer glucose tolerance and reduced diet-induced thermogenesis versus morning intake. In sum, while multiple observational analyses associate breakfast omission with higher BMI and adverse metabolic profiles, these designs cannot establish causality. Recent trials suggest that cardiometabolic improvements relate more robustly to the timing and consolidation of the eating window (especially earlier-day intake) than to breakfast per se. Thus, we avoid prescriptive ‘breakfast is essential’ claims and instead emphasize earlier, consolidated eating windows and avoidance of late-night intake as testable, circadian-concordant strategies. See Table 1 for an RCT-prioritized summary. Heterogeneity across studies on breakfast omission likely reflects contextual and methodological differences. Cultural eating patterns (for example, populations where breakfast is infrequently consumed), varying operational definitions of ‘breakfast’ (timing, energy threshold, composition), and energy compensation later in the day (especially late-night intake) can all modify associations. Individual factors including chronotype, sleep duration/quality, shift work, baseline adiposity and metabolic status, and melatonin-related glucose physiology further shape risk profiles. Study design (observational vs. randomized), dietary assessment methods, weekday–weekend differences, and socioeconomic and physical activity confounders also contribute to discordant findings. These findings imply that encouraging regular breakfast consumption may be advantageous for weight management and overall health.
Table 1.
Summary of selected human studies investigating the effects of meal timing on metabolic health
| S/N | Study and year | Design | Population | Timing of intervention | Intervention details (Dose/Calories/Nutrient) | Key findings | Effect size |
|---|---|---|---|---|---|---|---|
| 1 | Jakubowicz et al. [55] (2013) | Randomized controlled trial | Women with obesity (n = 93) | High-calorie breakfast vs. dinner for 12 weeks | ~ 1400 kcal/day, with either a large breakfast (700/500/200 kcal for breakfast/lunch/dinner) or a large dinner (200/500/700 kcal) | Greater weight loss, improved insulin and ghrelin profiles with high-calorie breakfast | ~ 2.5 kg weight loss; ↓ insulin by 33% |
| 2 | Sutton et al. [11] (2018) | Randomized crossover trial | Men with prediabetes (n = 8) | eTRE (6-hr feeding period, with dinner before 3 p.m.) vs. a control schedule (12-hr feeding period) for 5 weeks | Macronutrient details: 50% carbs, 35% fat, 15% protein with 3 meals/day, each ~ 1/31/3 of daily calories | Improved insulin sensitivity, blood pressure, and oxidative stress in eTRE group | ↑ insulin sensitivity by ~ 25% |
| 3 | Gabel et al. [46] (2018) | Pilot clinical trial | Obese adults (n = 23) | 8-hour TRE (10 a.m.–6 p.m.) for 12 weeks. | Participants ate without restrictions on type or amount of food) between 10:00 to 18:00 each day. Water fasting between 18:00 to 10:00 h) | Weight loss, lower insulin levels, improved eating behavior | ~ 3% weight loss; ↓ insulin 11% |
| 4 | Jamshed et al. [52] (2019) | Randomized crossover trial | Overweight adults (n = 11) | eTRE vs. mid-day feeding, that is, 8 am and 2 pm eTRF and between 8 am and 8 pm (control schedule) for 4 days | No report of total caloric intake or energy content of meals | Lower 24-h glucose and improved autophagy markers in eTRE group | ↓ glucose AUC by ~ 10% |
| 5 | McHill et al. [23] (2017) | Observational study | Healthy adults (n = 110) | Late eating vs. early eating for 30 days. On average, nonlean individuals consumed most of their calories about 1.1 h closer to melatonin onset than lean individuals | The study did not focus on a specific intervention dose or prescribed calorie intake; rather, it tracked natural, habitual consumption. | Later eating associated with higher body fat and lower energy expenditure | ↑ body fat by ~ 1.2 kg in late eaters |
| 6 | Bandin et al. [56] (2015) | Crossover trial | Healthy adults (n = 32) | Lunch at 13:00 (Early Eating) vs. 16:30 (Late Eating) | Caloric amount and nutrient composition of the meals were not detailed. Duration was 4 weeks | Evening meals impaired glucose tolerance and lipid metabolism | ↓ glucose tolerance by ~ 15% |
| 7 | Wilkinson et al. [57] (2020) | Clinical intervention study | Metabolic syndrome patients (n = 19) | Eating window restricted to 10 h/day (TRE; 8 a.m.–6 p.m.), 12-week duration | No macronutrient or micronutrient data provided; no caloric targets or dose reported. | Weight reduction, improved BP, LDL, and adherence to feeding window | ~ 3 kg weight loss; ↓ BP by 7 mmHg |
| 8 | Garaulet et al. [58] (2013) | Prospective cohort study | Participants with obesity (n = 420) | Lunch before 15:00 (early) vs. after 15:00 (late) 20-week weight-loss treatment | No difference in macronutrient composition; no dosed intervention | Early lunch eaters lost more weight and responded better to dietary interventions | ↑ weight loss success by ~ 30% |
| 9 | Potter et al. [59] (2016) | Review and mechanistic synthesis | General adult population | General feeding-window restriction in animals | Specific dose/calories values not reported | Meal timing impacts hormonal rhythms and metabolic regulation | Not applicable |
| 10 | Rubio-Sastre et al. [60] (2014) | Randomized crossover trial | Healthy volunteers (n = 21) |
Morning vs. evening glucose challenge with melatonin. 75 g glucose administered 15 min before OGTT; tests conducted at 9 AM and 9 PM |
Melatonin 5 mg (liquid form, ~ 0.9 mL); 75 g glucose. Duration was 1 day. | Evening melatonin intake impaired glucose tolerance compared to morning | ↓ glucose tolerance by ~ 18% |
| 11 | Reutrakul & Knutson [61] (2015) | Observational review | Adults with variable sleep/eating patterns | Observed real-world eating/sleeping behaviors | There are no specific doses, caloric counts, nutrient breakdowns, or precise timing regimens outlined in this review | Late eating and sleep disruption linked to increased obesity and poor metabolic health | Not quantified |
Arrows indicate the direction of change in epigenetic marks:↓ = decrease or reduction↑ = increase or elevation
4.2. Late-night eating and weight gain
Nighttime eating has emerged as a key contributor to obesity, particularly in shift workers and individuals with delayed sleep-wake schedules. Eating during the biological night is misaligned with the circadian rhythm of metabolism, which favors nutrient storage over utilization. Studies in humans have demonstrated that consuming a high-calorie meal at night leads to reduced diet-induced thermogenesis and greater postprandial glucose excursions compared to identical meals consumed in the morning [8]. Figure 2 is a brief comparison of metabolic responses to meals consumed at different times. Furthermore, delayed eating has been shown to suppress melatonin onset and interfere with lipid oxidation, promoting visceral fat accumulation [38]. These effects are especially pronounced in populations with evening chronotypes, who are more likely to consume larger meals later in the day. A longitudinal prospective cohort study involving 9,474 Korean adults with an average age of 54 years found that consuming midnight snacks and increased energy intake from these snacks correlated with a heightened risk of obesity, whereas sleeping 8 h or more was linked to a reduced risk of obesity [39]. It is also noteworthy that late-night eating often coincides with shorter sleep duration and poorer sleep quality. Insufficient sleep independently alters appetite-regulating hormones by increasing ghrelin and reducing leptin, thereby promoting hyperphagia and preference for calorie-dense foods. Sleep curtailment also impairs insulin sensitivity and glucose tolerance, compounding the adverse effects of nocturnal eating. Thus, part of the association between night eating and obesity may be mediated or amplified by concurrent sleep disruption, highlighting the intertwined roles of meal timing and sleep in circadian-metabolic health. Additionally, consuming late-night meals may lead to weight increase and hyperglycemia, thereby worsening cardiac disease [40, 41]. Consuming food outside of standard daytime hours can interfere with circadian rhythms, impacting metabolic efficiency and the regulation of appetite hormones [42]. The precise correlation between nocturnal consumption and obesity is ambiguous; nonetheless, data indicates that specific individuals may be more vulnerable to weight gain associated with midnight eating [43].
Fig. 2.
Comparison of metabolic responses to meals consumed at different times. Early meals are associated with higher insulin sensitivity, improved glucose tolerance, and greater lipid oxidation, while late meals reduce metabolic efficiency and increase adiposity risk. These comparisons are based on studies where calorie content and macronutrient composition were held constant, isolating the effect of timing. However, in free-living populations, late-night meals are also more likely to consist of energy-dense, nutrient-poor foods (like snacks or fast food), which may further exacerbate adverse metabolic effects
4.3. Time-restricted eating (TRE)
Time-restricted eating (TRE) is a circadian-aligned approach that limits daily food intake to a consistent time window (typically 6 to 10 h) without necessarily reducing caloric intake. TRE primarily targets circadian synchronization, aiming to align food consumption with the body’s active phase and fasting during the biological rest phase. TRE has demonstrated promising metabolic benefits [44]. Although some human trials report no change in daily energy intake when meals are confined to a restricted window, others observe a modest spontaneous reduction in calories, largely due to decreased late-evening snacking. Importantly, metabolic improvements such as enhanced insulin sensitivity and blood pressure reduction have been documented even in the absence of caloric deficit, suggesting that both timing and energy intake contribute to TRE’s effects. However, multiple RCTs report similar outcomes irrespective of whether the eating window is placed early or later when the window length and energy intake are matched. Neutral timing effects have been reported for weight loss, fasting glucose, HbA1c, and lipids in several studies, while a subset shows glycemic advantages for eTRE (particularly in prediabetes). Thus, the size and consistency of the eating window and avoidance of late-night intake may be more important than clock-time placement for many endpoints, with eTRE showing a plausible but not universal edge for postprandial glycemia. In rodent studies, TRE without caloric restriction protected against high-fat-diet-induced obesity, hepatic steatosis, and glucose intolerance [45]. These studies provide mechanistic insights into circadian alignment, showing enhanced mitochondrial function, reduced inflammation, and restored hormonal rhythms. In human trials, early time-restricted feeding (eTRE), which concentrates the eating window earlier in the day (e.g., 8 a.m. to 4 p.m.), was associated with reduced insulin levels, improved insulin sensitivity, and lower blood pressure, even without weight loss [11]. These benefits appear to be mediated by enhanced mitochondrial function, improved substrate oxidation, reduced inflammation, upregulation of autophagy during fasting hours, and restored hormonal rhythms (e.g., insulin, leptin, ghrelin) [46, 47]. However, adherence is variable, and study durations remain short (typically ≤ 12 weeks). The contrast highlights that while animal models confirm biological plausibility, translation to sustainable human interventions requires consideration of cultural, behavioral, and occupational constraints. A systematic study indicated that TRE resulted in an average weight reduction of 3% and diminished fat mass, independent of caloric restriction [48]. TRE has demonstrated advantageous metabolic advantages irrespective of weight loss, indicating an inherent impact derived from synchronizing meals with the circadian rhythm [48]. TRE is a potential approach for preventing and treating metabolic disorders; however, further high-quality research are required to evaluate its effectiveness across various populations and conditions [49].
4.4. Intermittent fasting and chrononutrition
Intermittent fasting (IF) encompasses broader regimens such as alternate-day fasting (ADF), the 5:2 diet (two non-consecutive fasting days per week), and periodic prolonged fasting (>24 h). IF involves complete or substantial caloric restriction for defined intervals, often interspersed with ad libitum feeding periods. IF may exert metabolic effects through both caloric restriction and circadian realignment, as illustrated in Fig. 3. Studies show that IF improves lipid profiles, reduces oxidative stress, induces autophagy and cellular repair, although outcomes vary depending on the timing and duration of the fast [8, 50]. The effects of IF are strongly influenced by total energy deficit and the duration of fasting rather than precise alignment with the circadian cycle. Emerging data suggest that combining intermittent fasting with early-day feeding enhances metabolic flexibility and may be more effective than evening fasting strategies [51]. Notably, a 4-day randomized crossover trial comparing early versus mid-day fasting windows found superior glycemic and lipid outcomes in the early group [52]. In these controlled trials, caloric intake and macronutrient composition were matched, underscoring that the observed benefits were attributable to fasting–feeding timing rather than diet composition. However, in real-world settings, food choices often differ by time of day, with earlier meals typically higher in complex carbohydrates and proteins, and later meals more likely to include energy-dense, nutrient-poor foods. This suggests that both the timing and the quality of food consumed may interact to shape the metabolic effects of intermittent fasting. The efficacy of intermittent fasting (IF) relative to conventional diets is still contentious, with results primarily contingent upon caloric restriction [53]. Research in rats has shown that disturbance of tissue-specific circadian rhythms can lead to obesity or impair glucose homeostasis [8].
Fig. 3.
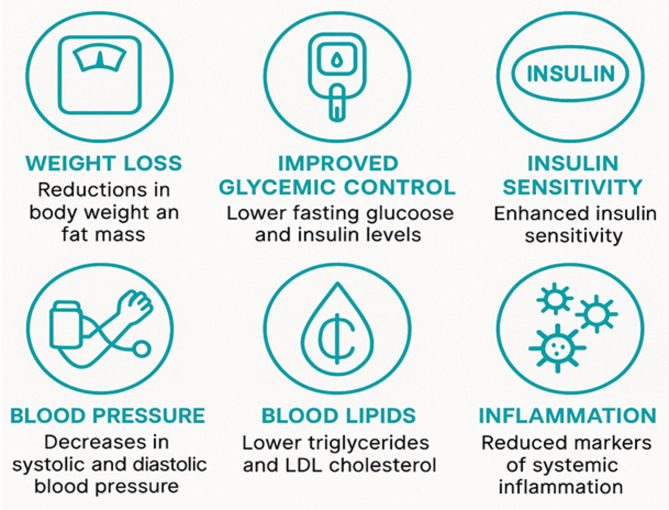
Summary of clinical outcomes reported in trials of time-restricted eating (TRE) and intermittent fasting. Benefits include reductions in body weight and fat mass, improved glycemic control, enhanced insulin sensitivity, lowered blood pressure, improved lipid profiles, and decreased systemic inflammation
From a behavioral standpoint, TRE is generally easier to maintain due to its daily consistency and flexibility in food choice, while IF often requires more intensive dietary control and may lead to compensatory eating behaviors on non-fasting days [54]. Additionally, TRE appears to offer greater circadian alignment, especially when practiced as early-TRE (eTRE), while IF’s metabolic effects are more dependent on the magnitude of caloric restriction and fast duration rather than meal timing. While both strategies exhibit promises in improving cardiometabolic health, their differential impacts underscore the need for personalized recommendations based on individual chronotype, lifestyle constraints, and metabolic profiles. Figure 3 provides a summary of clinical outcomes for both approaches. Table 1 is a summary of selected human studies investigating the effects of meal timing on metabolic health. Outcomes reflect changes in insulin sensitivity, weight loss, glucose tolerance, and cardiometabolic risk under various temporal feeding interventions. Details on intervention timing and specific intervention characteristics (e.g., melatonin dose, caloric intake, meal composition) are included where available.
4.5 summary of recent clinical advances and critical appraisal (2019–2025)
In the past five years, several high-quality clinical trials and observational studies have strengthened the evidence linking meal timing with metabolic health. For example, Lyu et al. [39] demonstrated in a large prospective cohort that midnight snacking significantly increased obesity incidence, whereas adequate sleep duration mitigated this risk. Similarly, Díaz-Rizzolo et al. [40] found that late eating worsens glucose tolerance independently of body weight and diet composition, underscoring a timing-specific effect. Controlled trials of TRE, such as Wilkinson et al. [57] and Sutton et al. [11], consistently report improvements in insulin sensitivity and blood pressure without weight loss, suggesting metabolic benefits beyond caloric restriction.
However, findings remain heterogeneous. For example, while Ma et al. [35] reported a 48% increased risk of obesity with breakfast skipping, longitudinal analyses by Wicherski et al. [37] showed only modest associations, indicating potential confounding by lifestyle factors. Many studies are also limited by small sample sizes (often < 50 participants), short intervention durations (4–12 weeks), and variable definitions of “early” vs. “late” eating windows. These inconsistencies highlight the need for standardization and longer follow-up.
Taken together, recent evidence reinforces the hypothesis that meal timing independently influences obesity risk, but also reveals methodological weaknesses that limit generalizability. A key research priority is establishing whether circadian-aligned eating patterns remain effective and sustainable in diverse real-world settings over the long term.
5. Nutrient timing and metabolic outcomes
While total energy intake and macronutrient balance are central to metabolic regulation, the timing of nutrient consumption across the day exerts additional and often underappreciated influence on body weight, hormonal rhythms, and substrate metabolism. Nutrient timing not only impacts the magnitude of postprandial responses but also modulates circadian regulation of metabolic pathways, potentially contributing to or mitigating obesity and its complications [8]. An important consideration is whether differences in the types of nutrients typically consumed at morning versus evening meals might themselves explain some of the metabolic outcomes observed. Morning meals are often richer in carbohydrates and protein, while evening meals tend to include more fats and alcohol, depending on cultural context. These patterns align with physiological rhythms: carbohydrate tolerance and insulin sensitivity are highest in the morning, whereas fat oxidation is less efficient at night. Emerging evidence also suggests that certain micronutrients, such as vitamin D, zinc, and dietary fiber, may be disproportionately consumed during earlier meals, potentially contributing to improved satiety, glycemic control, and immune-metabolic regulation [62]. Thus, both macronutrient and micronutrient distributions across the day could interact with circadian biology, amplifying or attenuating the effects of meal timing. More research is required to disentangle the independent effects of timing from those of nutrient composition.
5.1. Macronutrient distribution across the day
There is increasing evidence that the distribution of macronutrients including carbohydrates, fats, and proteins across the circadian cycle affects metabolic efficiency. Several studies have demonstrated that carbohydrate tolerance is higher in the morning and declines throughout the day due to diurnal variations in insulin sensitivity and glucose oxidation [63]. Consequently, high-carbohydrate meals consumed in the evening are more likely to result in postprandial hyperglycemia and lipogenesis than those consumed earlier in the day [64]. A randomized crossover trial by Jakubowicz et al. [55] found that women with obesity assigned to a high-calorie breakfast (700 kcal) with lower-calorie dinners achieved significantly greater weight loss and better insulin and ghrelin profiles than those consuming the same total calories with a high-calorie dinner. Similar findings have been observed with protein distribution, where morning protein consumption was more effective in reducing appetite and preserving lean mass than evening protein intake [65]. These results suggest that synchronizing macronutrient intake with diurnal metabolic capacity may enhance the efficacy of dietary interventions.
A systematic analysis indicated that those with good sleep exhibited greater energy allocation from dietary protein than those with bad sleep [66]. The circadian clock system affects nutrition metabolism, impacting postprandial responses to macronutrients according to the time of day [67]. The timing and frequency of meals throughout the day are linked to metabolic processes, indicating that optimal time-restricted feeding may avert metabolic dysfunctions. These findings underscore the significance of evaluating macronutrient distribution and timing concerning many health outcomes.
5.2. Chrononutritional interactions with hormones
Chrononutrition, a nascent discipline under chronobiology, investigates the relationships among circadian rhythms, metabolic processes, and dietary intake [8]. The timing of food consumption, physical activity, and stress can influence circadian rhythms and the expression of clock genes in peripheral organs [68]. Ketone bodies, beyond serving as energy substrates, act as signaling molecules that influence circadian-regulated processes. For example, β-hydroxybutyrate can modulate histone acetylation, thereby altering the expression of core clock genes (BMAL1, PER2) and downstream metabolic pathways. Elevated ketones during fasting or carbohydrate restriction have been shown to reinforce circadian oscillations in appetite-regulating hormones, dampen nocturnal ghrelin surges, and stabilize leptin rhythms. Moreover, ketone signaling interacts with sleep–wake regulation through modulation of GABAergic and glutamatergic pathways, linking nutrient availability to circadian control of hormone secretion [69]. The principles of chrononutrition have demonstrated potential in enhancing weight loss and metabolic health in humans [8]. Moreover, chronodisruption, resulting from irregular eating patterns or shift employment, has been associated with numerous health complications, including metabolic syndrome and mental health difficulties [70]. Customised chrononutrition approaches present opportunities for the prevention and management of chronic health disorders [70]. Recent studies have emphasised the impact of circadian rhythms on essential metabolic tissues and hormone secretion [69]. Hormonal regulators of hunger, satiety, and energy storage, such as insulin, leptin, ghrelin, cortisol, and melatonin, follow circadian rhythms and interact closely with feeding behavior. Disrupted timing of nutrient intake can desynchronize these hormonal patterns, exacerbating metabolic disturbances. For instance, ghrelin, which stimulates hunger, exhibits pre-meal surges and is suppressed by regular eating patterns; irregular or delayed meals may blunt ghrelin cycling, increasing the risk of overeating [71]. Furthermore, leptin, secreted predominantly at night, signals satiety and energy sufficiency. Late-night eating interferes with leptin signaling and may promote positive energy balance [72]. Ghrelin, by contrast, peaks prior to meals and drives hunger; irregular or skipped meals can blunt its rhythmicity, leading to overeating later in the day. Similarly, leptin resistance, often seen with obesity, may weaken satiety cues, particularly when meals are mistimed [73]. These hormonal interactions help explain why consistent meal timing enhances appetite regulation, while misaligned or irregular eating exacerbates metabolic risk.
On the other hand, cortisol, with its early morning peak, enhances gluconeogenesis and lipolysis. Nutrient timing that aligns with this hormonal peak may facilitate more efficient energy metabolism [74]. Melatonin, secreted in the evening, reduces pancreatic β-cell responsiveness and thereby impairs insulin secretion and glucose tolerance. Late-evening food intake coincides with this physiological decline, worsening postprandial glucose handling. Importantly, individuals carrying common variants in the melatonin receptor gene MTNR1B show heightened susceptibility: elevated melatonin at night or disrupted melatonin rhythms have been linked to impaired glucose tolerance, increased fasting glucose, and greater risk of prediabetes and type 2 diabetes. These findings suggest that both physiological nocturnal melatonin peaks and circadian misalignment contribute causally to metabolic imbalance [75]. This interplay between nutrient intake and hormonal rhythms underscores the importance of temporal coordination in nutrition to optimize hormonal signals and metabolic outcomes.
5.3. Sleep, circadian misalignment, and nutrition
Nutrition, sleep, and circadian timing are deeply interconnected. Inadequate or mistimed sleep alters hunger-regulating hormones, promoting hyperphagia and weight gain [76]. Sleep deprivation increases ghrelin, decreases leptin, and enhances cravings for calorie-dense foods, particularly when meals are consumed at night [77]. Disruptions in sleep and circadian rhythms are recognised as emerging risk factors for obesity, especially when they manifest throughout infancy and early adulthood [78].
Shift work, which imposes circadian misalignment between behavioral and biological rhythms, has been strongly linked to increased body mass index (BMI), insulin resistance, and metabolic syndrome [79]. Importantly, misaligned nutrient intake during the rest phase, common in night-shift workers, has been shown to impair glucose metabolism independently of total sleep duration. This effect is partly attributable to circadian regulation of core body temperature, which peaks in the late afternoon and declines at night. Eating during the low-temperature phase blunts diet-induced thermogenesis and reduces insulin sensitivity, thereby compounding the adverse metabolic effects of nocturnal food intake [23]. Moreover, circadian misalignment may blunt the thermic effect of food, alter gut microbiota composition, and reduce brown adipose tissue activation, which are mechanisms that together contribute to obesogenic metabolic profiles [80]. This highlights the need for dietary timing strategies that are synchronized with sleep-wake cycles and endogenous circadian physiology.
Previous research has investigated the correlation between social jetlag and metabolic health. Social jetlag refers to the misalignment between an individual’s internal biological clock and externally imposed social schedules (such as later sleep/wake patterns on weekends compared to workdays). This discrepancy has been linked to a heightened risk of obesity and metabolic disorders [81, 82]. Social jetlag has been associated with heightened fasting glucose levels and tendencies to overweight [82] and a greater propensity for developing metabolic syndrome [83]. Social jetlag has been examined for its possible effects on glycaemic and metabolic regulation in individuals with type 2 diabetes [84]. The findings indicate that living contrary to one’s circadian rhythm may lead to metabolic dysfunction and its repercussions, underscoring the necessity of addressing social jetlag in the prevention of obesity and the management of metabolic health. Optimal metabolic outcomes depend not only on what is eaten, but when it is eaten. Aligning macronutrient intake with the body’s metabolic peaks, considering hormonal profiles, and supporting circadian rhythms through synchronized sleep and feeding cycles offer a promising, non-pharmacological approach to combat obesity and metabolic disease. Future personalized nutrition plans should incorporate these chronobiological principles to enhance long-term efficacy.
6. Clinical and public health implications
The emerging evidence on circadian nutrition underscores the importance of integrating meal timing into strategies for the prevention and management of obesity and its metabolic comorbidities. Beyond individual clinical applications, circadian-based interventions have the potential to inform public health policy, guide workplace wellness programs, and shape dietary guidelines.
6.1. Integrating circadian nutrition in obesity management
Current dietary recommendations often focus on what and how much to eat, with minimal attention to when food is consumed. Incorporating circadian principles into clinical practice may enhance weight loss outcomes and metabolic health, particularly in individuals with obesity, type 2 diabetes, or metabolic syndrome [57]. Approaches such as early time-restricted eating (eTRE) or simply shifting caloric intake toward the earlier part of the day have shown benefits in reducing insulin resistance, blood pressure, and appetite dysregulation, even without weight loss [11]. Personalized dietary advice that accounts for an individual’s chronotype (their natural biological preference for morning or evening activity), work schedule, and sleep patterns may further optimize results. For example, evening chronotypes could benefit from gradual realignment of meal times, while shift workers may require targeted counseling to minimize nighttime eating during work hours [85].
6.2. Challenges in real-world application
A key conceptual question is whether the observed benefits of chrononutrition simply reflect inadvertent caloric restriction or whether meal timing exerts independent effects. Evidence increasingly supports the latter. For instance, Sutton et al. [11] reported improvements in insulin sensitivity, blood pressure, and oxidative stress in men with prediabetes practicing early time-restricted feeding, despite no significant weight loss. Likewise, animal models demonstrate that aligning feeding to the active phase protects against obesity and metabolic dysfunction even under isocaloric conditions. Notably, very few human trials have employed strictly isocaloric designs when testing time-restricted eating. In contrast, several animal studies have rigorously controlled caloric intake, providing clearer evidence of independent circadian effects. For instance, Hatori et al. [45] demonstrated that mice fed a high-fat diet within a restricted feeding window were protected against obesity, hepatic steatosis, and glucose intolerance, despite consuming the same total calories as ad libitum controls. Similar rodent experiments confirm that aligning feeding to the active phase preserves insulin sensitivity, optimizes mitochondrial function, and prevents fat accumulation under isocaloric conditions. These findings support the concept that the benefits of chrononutrition extend beyond energy reduction and are mediated by circadian alignment itself. However, the translation of these results to humans remains limited, underscoring the urgent need for carefully designed isocaloric trials in clinical populations. These findings highlight that while reduced energy intake may occur in real-world practice, chrononutrition is not merely a proxy for calorie restriction but instead represents a distinct strategy that harnesses circadian physiology to improve metabolic outcomes. Despite promising results, the real-world implementation of circadian-based dietary interventions faces several challenges:
-
i.
Cultural norms: In many cultures, dinner is the largest and latest meal, making early caloric loading socially challenging. Cultural eating patterns, especially in Southern Europe, Asia, and Latin America, favor late-night dinners, making adherence to early TRE difficult [86, 87]. Instead of broad restructuring of social norms, practical strategies may include gradual temporal shifts (e.g., moving dinner earlier by 30–60 min), community-based education, or tailoring interventions to local traditions.
-
ii.
Work schedules and social jet lag: Irregular or extended work hours, especially among shift workers, make consistent meal timing difficult [59]. Here, interventions should focus on minimizing nocturnal eating during biological night rather than eliminating it entirely, as supported by recent RCTs simulating night work.
-
iii.
Behavioral adherence: Behavioral adherence is another major barrier. Time-restricted eating requires sustained effort, and real-world studies show variable compliance. Time-restricted eating and other temporal interventions require sustained behavioral change, which may be difficult to maintain without appropriate support systems. Additionally, behavioral inertia and a lack of awareness among clinicians and patients contribute to limited uptake of meal-timing strategies. Digital health tools, such as mobile applications that track feeding windows and provide reminders, may assist in promoting adherence and lifestyle modification [46, 88].
-
iv.
Social influences: Social influences including family routines, social events, and mealtimes, may not align with circadian-aligned eating. Digital tools, including mobile apps and wearables, have demonstrated potential in reinforcing feeding windows and improving adherence [89, 90]. Recognizing these barriers rather than assuming structural change is feasible in the short term is crucial for designing realistic, scalable interventions. Additionally, public health campaigns should educate the population about the benefits of aligning eating patterns with biological rhythms.
6.3. Real-world relevance, equity, and global applicability
While the scientific evidence supporting circadian nutrition is compelling, its real-world application must consider sociocultural, economic, and structural factors that influence eating behavior across populations. Implementing circadian-aligned dietary strategies in routine care or public health programming requires sensitivity to cultural norms, occupational patterns, and health disparities that shape food access and timing, as highlighted below.
-
i.
Cultural adaptability: In many cultures, particularly in Southern Europe, Latin America, Asia, and parts of Africa, late-evening meals are customary and socially embedded. Thus, rigid adherence to eTRE may face resistance or prove unsustainable. Interventions must therefore be culturally adaptable, encouraging gradual temporal shifts while respecting traditional dietary patterns. For example, trials in Southern European cohorts have shown that progressively shifting the timing of the main meal earlier in the day improved weight-loss outcomes compared with abrupt changes [87]. Similarly, studies in obesity interventions by Ruddick-Collins et al. [32] and adherence-focused trials [54] demonstrate that gradually narrowing the eating window over several weeks enhances compliance. In shift-work simulations, stepwise realignment of meals toward the biological day has likewise been effective in mitigating circadian disruption [91]. These findings suggest that cultural and behavioral feasibility is improved when temporal realignment is gradual rather than rigidly imposed. Community-level sensitization and stakeholder engagement are critical to fostering behavioral change.
-
ii.
Health equity and vulnerable populations: Individuals in low-resource settings, shift workers, and populations with lower socioeconomic status often experience “chrononutritional inequity”, limited control over meal timing due to structural constraints such as irregular work schedules, food insecurity, or lack of access to healthy options during daytime hours. These disparities must be acknowledged in both clinical practice and policy design to avoid exacerbating health inequalities. Circadian-aligned strategies should be tailored to context, promoting flexible time windows and leveraging existing community structures.
-
iii.
Global health integration: Despite the global rise in obesity, circadian nutrition remains underrepresented in dietary guidelines. Chrononutrition is not yet integrated into dietary guidelines primarily because most studies are short-term, heterogeneous in design, and lack long-term adherence and safety data. Nevertheless, several practices are supported by converging evidence. Front-loading caloric intake to earlier in the day improves insulin sensitivity, satiety, and weight outcomes in randomized controlled trials. Avoiding late-night eating, particularly after ~ 9 p.m., consistently prevents metabolic impairment across both experimental and cohort studies. In contrast, the evidence on breakfast skipping remains inconsistent, with cultural and behavioral factors strongly modifying associations. As such, the most actionable evidence for policy translation at present lies in encouraging earlier energy intake and discouraging late-night meals, while further trials are needed before broader chrononutrition guidance can be standardized. Future integration should involve cross-sector collaboration between chronobiologists, nutritionists, behavioral scientists, and policymakers. Digital tools, mobile health interventions, and workplace programs may offer cost-effective solutions to monitor and optimize meal timing in diverse environments.
-
iv.
Equity: Equity-oriented public health messaging must shift the focus from individual responsibility to systemic enablers, such as school and work policies, urban planning (lighting, transportation), and institutional food provision which align daily life with biological rhythms.
6.4. Recommendations for specific populations
Clinical recommendations should balance biological plausibility with practical feasibility. Broad guidance includes encouraging earlier caloric intake, concentrating food within a 10–12-hour daytime window, and avoiding late-night eating. However, recommendations must be tailored:
-
i.
Chronotype: Morning types may benefit more from eTRE, while evening types may require gradual realignment rather than abrupt shifts.
-
ii.
Shift workers: Counseling should emphasize minimizing caloric intake during the biological night and aligning meals with active work phases.
-
iii.
Older adults: Structured meal timing may help stabilize circadian amplitude and reduce sarcopenia risk, but adjustments must consider mobility, medication schedules, and sleep disruptions.
Table 2 summarizes core, practical clinical recommendations. Unlike rigid prescriptions, these should be applied flexibly, tailored to patient chronotype, work schedules, and cultural context. These guidelines reflect both biological and behavioral considerations for optimizing metabolic outcomes. Certain populations may derive unique benefits from circadian nutrition strategies:
Table 2.
Clinical recommendations for integrating circadian nutrition in practice
| S/N | Domain | Recommendation | Rationale |
|---|---|---|---|
| 1 | Meal Timing | Encourage early caloric intake; concentrate food intake within a 10–12-hour daytime window. | Enhances insulin sensitivity and aligns nutrient metabolism with circadian rhythms. |
| 2 | Chronotype Consideration | Tailor feeding schedules to individual chronotypes; morning/evening preference. | Improves adherence and metabolic responsiveness by respecting individual biological clocks. |
| 3 | Macronutrient Timing | Consume carbohydrates and larger meals earlier in the day; reserve lighter meals for evening. | Reduces postprandial glucose spikes; improves thermogenesis; supports satiety. |
| 4 | Shift Work Adaptation | Minimize nighttime eating; align meals with active phases even during night shifts. | Mitigates circadian misalignment and reduces risk of obesity and metabolic syndrome. |
| 5 | Sleep and Feeding Synchrony | Promote consistent sleep and feeding patterns to maintain circadian synchrony. | Prevents hormonal desynchronization and supports appetite regulation. |
| 6 | Public Health Messaging | Integrate timing of meals into dietary guidelines and obesity prevention campaigns. | Expands public awareness of ‘when to eat’ as a modifiable factor in health. |
| 7 | Clinical Nutrition Practice | Include meal timing assessments in dietary intake forms and nutrition counseling. | Improves individualized care and increases clinician awareness of temporal patterns. |
| 8 | Digital and Behavioral Tools | Use mobile apps, wearables, and reminder systems to reinforce time-based eating. | Facilitates adherence and tracking of feeding windows in real-world settings. |
| 9 | Cultural and Dietary Norms | Respect traditional meal structures while gradually shifting caloric intake earlier in the day. | Enhances cultural acceptability and long-term sustainability of circadian interventions. |
| 10 | Pediatric and Adolescent Nutrition | Establish regular breakfast habits and limit late-night snacking to prevent early metabolic disruption. | Supports healthy growth and reduces risk of obesity and type 2 diabetes in youth. |
| 11 | Elderly and Chronically ill Populations | Adjust eating windows based on mobility, medication schedules, and sleep disruptions. | Accounts for biological changes, comorbidities, and reduced circadian robustness. |
| 12 | Policy and Worksite Interventions | Promote structured mealtimes and restrict food availability during night hours in institutional settings. | Shifts environmental cues toward healthier temporal eating behaviors in workplaces and schools. |
-
i.
Children and adolescents: Early establishment of regular meal timing may prevent the development of obesity and metabolic dysfunction. Aligning school meal schedules and avoiding late-night snacking may support healthy growth and metabolic programming [92]. However, to maintain an adult clinical scope, pediatric studies and recommendations are not used to support causal claims in this review.
-
ii.
Shift workers: Tailored interventions to minimize nocturnal eating and promote fasting during the biological night could mitigate cardiometabolic risks [91]. Implementing modified TRE schedules, optimizing nutrient timing during active work phases, and managing light exposure may mitigate circadian misalignment [93].
-
iii.
Older adults: Aligning feeding patterns with preserved circadian rhythms may help reduce sarcopenic obesity and metabolic syndrome [94]. Circadian amplitude often weakens with age. Structured meal timing may help stabilize rhythms and reduce sarcopenia risk [95].
-
iv.
Individuals with diabetes: Synchronizing meal timing with periods of higher insulin sensitivity (earlier in the day) could improve glycemic control [91].
Importantly, interventions should be presented as flexible frameworks rather than rigid prescriptions. Policies promoting earlier school lunches, workplace wellness programs, and digital adherence tools are more feasible than wholesale restructuring of social norms. By grounding clinical recommendations in both evidence and feasibility, circadian nutrition can be realistically integrated into patient care and public health initiatives.
7. Knowledge gaps and future directions
Despite a proliferation of studies in the past decade, a clear research gap remains at the interface of mechanistic insights and translational application. Most evidence supporting circadian nutrition comes from short-term interventions (< 12 weeks) with relatively homogenous populations, leaving questions about long-term sustainability, adherence, and population diversity unresolved. Importantly, while circadian biology suggests that chronotype and genetic variation strongly influence responsiveness to meal timing, few trials stratify participants accordingly. Likewise, the role of the gut microbiota in mediating circadian-metabolic interactions remains underexplored, with only preliminary evidence linking disrupted microbial oscillations to obesity risk.
Furthermore, despite compelling evidence, official dietary guidelines worldwide continue to omit circadian considerations, focusing primarily on caloric balance and macronutrient composition. This represents a critical translational gap that your review addresses: synthesizing emerging mechanistic and clinical data to argue for the integration of meal timing into public health recommendations. By explicitly identifying this gap, the review distinguishes itself from prior general summaries and provides a roadmap for advancing the field.
-
i.
Lack of long-term human trials: Although short-term studies of time-restricted eating and meal timing have yielded promising results, the long-term sustainability, safety, and efficacy of these approaches remain unclear. Most existing trials last fewer than 12 weeks, limiting conclusions on chronic disease risk reduction or weight maintenance. Furthermore, heterogeneity in study design, duration, fasting windows, and caloric distribution complicates comparisons and meta-analyses [96]. There is a pressing need for well-powered, randomized controlled trials with longer durations (≥ 6 months) that evaluate both physiological and behavioral outcomes, including adherence, satiety, metabolic markers, and psychological effects.
-
ii.
Contribution of caloric restriction vs. circadian alignment: Another unresolved issue concerns the extent to which benefits of chrononutrition are attributable to reduced energy intake versus circadian alignment itself. While animal studies with isocaloric controls strongly suggest that temporal alignment exerts intrinsic metabolic benefits, such designs are rare in human research. Most clinical TRE interventions result in modest energy reduction, making it difficult to isolate the true effects of timing. Future translational research should build on the rigor of animal models by incorporating isocaloric conditions in randomized controlled trials, thereby clarifying whether benefits observed in humans stem from circadian alignment per se, caloric restriction, or their interaction. While some trials show improvements in metabolic markers under isocaloric conditions, others report modest spontaneous calorie reductions during time-restricted eating. Carefully designed long-term randomized controlled trials that control for caloric intake are needed to clarify whether temporal eating strategies exert independent effects, additive benefits, or synergistic interactions with energy restriction.
-
iii.
Role of chronotype and individual variability: Emerging evidence suggests that individual chronotype, a person’s natural sleep-wake preference, modulates the response to temporal eating interventions. Evening chronotypes may experience greater metabolic misalignment when adhering to early time-restricted feeding (eTRE), while morning types may benefit more substantially [97]. However, few studies stratify participants based on chronotype, sex, age, or genetic background. Personalized nutrition models that incorporate circadian preference, metabolic phenotype, and sleep-wake patterns may enhance intervention efficacy, yet these models remain underexplored.
-
iv.
Mechanistic pathways beyond hormonal rhythms: Although hormonal regulators like insulin, ghrelin, and cortisol are well studied in circadian nutrition, other mechanistic pathways, including mitochondrial dynamics, autophagy, redox signaling, and gut microbiota oscillations, remain incompletely characterized [98]. Understanding how meal timing affects these systems could provide novel targets for obesity treatment. In particular, the gut microbiota exhibits diurnal variation in composition and function, influenced by feeding cycles. Disrupted microbiota rhythms may contribute to metabolic endotoxemia and insulin resistance, yet human data linking meal timing to microbiota-mediated obesity pathways are still limited [99].
-
v.
Unresolved mechanistic paradoxes: Another important gap lies in explaining why behaviors such as skipping breakfast are consistently associated with increased obesity risk in observational studies, whereas structured TRE regimens which may also involve delaying or omitting breakfast often yield metabolic benefits. This paradox likely reflects differences in behavioral context, diet quality, and circadian alignment: unstructured breakfast skipping may shift energy intake to the evening, amplify cortisol-leptin misalignment, and promote overeating, whereas TRE imposes temporal regularity and restricts late-night intake. More mechanistic studies measuring cortisol, leptin, ghrelin, and satiety signaling under different timing protocols are needed to clarify these apparent contradictions.
-
vi.
Influence of nutrient distribution across the day: Another underexplored area is the contribution of nutrient composition at different eating occasions. Variations in the proportion of carbohydrates, protein, and fat, as well as micronutrients such as vitamin D, zinc, and fiber between morning and evening meals may account for part of the observed metabolic differences. Future studies should compare isocaloric diets with controlled macronutrient and micronutrient distributions across different times of day to determine whether metabolic benefits are driven primarily by timing, by nutrient quality, or by their interaction.
-
vii.
Social, cultural, and environmental considerations: Real-world application of circadian nutrition strategies requires contextual understanding of sociocultural norms, food availability, meal timing traditions, and occupational demands. Most intervention studies are conducted in high-income countries with structured daily schedules, which may not generalize to diverse global populations. Future studies should evaluate culturally tailored interventions, explore feasibility in low-resource settings, and assess the impact of digital health technologies for monitoring and modifying eating schedules [100].
-
viii.
Integration into public health and clinical guidelines: Despite mounting evidence, official dietary guidelines rarely address meal timing or circadian alignment. Establishing standardized definitions for eating windows, “early” vs. “late” meals, and duration thresholds for TRE is essential for regulatory clarity. Collaborations between chronobiologists, nutritionists, and policymakers will be crucial for evidence-based guideline development and implementation.
Conclusion
The convergence of chronobiology and nutritional science has revealed a critical yet underutilized axis in obesity management: circadian timing of food intake. Not only what we eat but also when we eat appears to influence metabolic health. This review highlights compelling evidence that not only the quality and quantity of food, but also the timing of consumption, significantly influences metabolic health, energy balance, and risk of obesity-related complications. Eating at biologically appropriate times, such as concentrating caloric intake earlier in the day and avoiding late-night eating, optimizes insulin sensitivity, lipid metabolism, and hormonal rhythms. Interventions like time-restricted eating (TRE) and early time-restricted feeding (eTRE) have shown promising effects on weight regulation and cardiometabolic risk, even in the absence of calorie restriction. These strategies align food intake with endogenous circadian cycles, thereby reinforcing metabolic homeostasis.
Despite these promising findings, real-world implementation remains challenged by cultural, occupational, and behavioral barriers. Furthermore, gaps in long-term data, individual variability, and mechanistic understanding highlight the need for more personalized and population-specific research. Moving forward, public health strategies and clinical guidelines must incorporate temporal dimensions of eating behavior to enhance the efficacy and sustainability of dietary interventions. While reductions in caloric intake may partly explain observed benefits, a growing body of evidence indicates that circadian-aligned eating exerts distinct metabolic effects independent of calorie reduction. However, because much of the current evidence comes from small, short-duration trials with heterogeneous designs, our conclusions are intentionally cautious and intended to guide hypothesis-driven clinical practice rather than assert definitive causality. Larger, longer, and more diverse RCTs are needed before strong recommendations can be made.
Acknowledgements
None.
Author contributions
Conceptualization: Esther Ugo Alum, Methodology: Esther Ugo Alum, Investigation: Esther Ugo Alum, Resources: Esther Ugo Alum, Supervision: Esther Ugo Alum, Validation: Esther Ugo Alum, Visualization: Esther Ugo Alum, Writing – original draft: Esther Ugo Alum, Writing – review & editing: Esther Ugo Alum. All authors reviewed the manuscript.
Funding
No funding was received.
Data availability
All data generated during this study are included in the manuscript.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Clinical trial registration
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alum EU, Ejemot-Nwadiaro RI, Betiang PA, Basajja M, Uti DE. Obesity and climate change: a two-way street with global health implications. Obes Med. 2025;100623. 10.1016/j.obmed.2025.100623. [Google Scholar]
- 2.Obesity. and overweight. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 3.Johnson VR, Washington TB, Chhabria S, Wang EH-C, Czepiel K, Reyes KJC, Stanford FC. Food as medicine for obesity treatment and management. Clin Ther. 2022;44:671–81. 10.1016/j.clinthera.2022.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panda S. Circadian physiology of metabolism. Science. 2016;354:1008–15. 10.1126/science.aah4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bass J, Lazar MA. Circadian time signatures of fitness and disease. Science. 2016;354:994–9. 10.1126/science.aah4965. [DOI] [PubMed] [Google Scholar]
- 6.Asher G, Sassone-Corsi P. Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell. 2015;161:84–92. 10.1016/j.cell.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 7.Zimmet PZ, Magliano DJ, Herman WH, Shaw JE. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. 2014;2:56–64. 10.1016/S2213-8587(13)70112-8. [DOI] [PubMed] [Google Scholar]
- 8.Song D-K, Kim Y-W. Beneficial effects of intermittent fasting: a narrative review. J Yeungnam Med Sci. 2022;40:4–11. 10.12701/jyms.2022.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alum E, Obeagu E, Ugwu O, Alum B, Echegu A, Ukaidi C. Differential Impacts of Intermittent Fasting on Men and Women. Elite Journal of Health Science. 2024; 2 37–44 .
- 10.Chaix A, Manoogian ENC, Melkani GC, Panda S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr. 2019;39:291–315. 10.1146/annurev-nutr-082018-124320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early Time-Restricted feeding improves insulin Sensitivity, blood Pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27:1212–e12213. 10.1016/j.cmet.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raji OE, Kyeremah EB, Sears DD, St-Onge M-P, Makarem N. Chrononutrition and cardiometabolic health: an overview of epidemiological evidence and key future research directions. Nutrients. 2024;16:2332. 10.3390/nu16142332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuad SA, Ginting RP, Lee M-W. Chrononutrition: potential, challenges, and application in managing obesity. Int J Mol Sci. 2025;26:5116. 10.3390/ijms26115116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Regmi P, Young M, Minigo G, Milic N, Gyawali P. Photoperiod and metabolic health: evidence, mechanism, and implications. Metabolism. 2024;152:155770. 10.1016/j.metabol.2023.155770. [DOI] [PubMed] [Google Scholar]
- 15.Sato S, Solanas G, Sassone-Corsi P, Benitah SA. Tuning up an aged clock: circadian clock regulation in metabolism and aging. Transl Med Aging. 2022;6:1–13. 10.1016/j.tma.2021.11.003. [Google Scholar]
- 16.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18:164–79. 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenwell BJ, Trott AJ, Beytebiere JR, Pao S, Bosley A, Beach E, Finegan P, Hernandez C, Menet JS. Rhythmic food intake drives rhythmic gene expression more potently than the hepatic circadian clock in mice. Cell Rep. 2019;27:649–e6575. 10.1016/j.celrep.2019.03.064. [DOI] [PubMed] [Google Scholar]
- 18.Schrader LA, Ronnekleiv-Kelly SM, Hogenesch JB, Bradfield CA, Malecki KMC. Circadian disruption, clock genes, and metabolic health. J Clin Invest. 2024. 10.1172/JCI170998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maury E, Navez B, Brichard SM. Circadian clock dysfunction in human omental fat links obesity to metabolic inflammation. Nat Commun. 2021;12:2388. 10.1038/s41467-021-22571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu R, Tian L, Ding Y, Gao Y, Li D, Tang Y. Correlation between inflammatory markers and impaired circadian clock gene expression in type 2 diabetes mellitus. Diabetes Res Clin Pract. 2019;156:107831. 10.1016/j.diabres.2019.107831. [DOI] [PubMed] [Google Scholar]
- 21.Samblas M, Milagro FI, Martínez A. DNA methylation markers in obesity, metabolic syndrome, and weight loss. Epigenetics. 2019;14:421–44. 10.1080/15592294.2019.1595297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaput J-P, McHill AW, Cox RC, Broussard JL, Dutil C, da Costa BGG, Sampasa-Kanyinga H, Wright KP. The role of insufficient sleep and circadian misalignment in obesity. Nat Rev Endocrinol. 2023;19:82–97. 10.1038/s41574-022-00747-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McHill AW, Phillips AJ, Czeisler CA, Keating L, Yee K, Barger LK, et al. Later circadian timing of food intake is associated with increased body fat. Am J Clin Nutr. 2017;106:1213–9. 10.3945/ajcn.117.161588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mason IC, Qian J, Adler GK, Scheer FAJL. Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia. 2020;63:462–72. 10.1007/s00125-019-05059-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wever MCM, van Meer F, Charbonnier L, Crabtree DR, Buosi W, Giannopoulou A, et al. Associations between ghrelin and leptin and neural food cue reactivity in a fasted and sated state. Neuroimage. 2021;240:118374. 10.1016/j.neuroimage.2021.118374. [DOI] [PubMed] [Google Scholar]
- 26.Macfarlane E, Zhou H, Seibel MJ. Endogenous glucocorticoids during skeletal ageing. Explor Endocr Metab Dis. 2024;1:191–212. 10.37349/eemd.2024.00016. [Google Scholar]
- 27.Garaulet M, Qian J, Florez JC, Arendt J, Saxena R, Scheer FAJL. Melatonin effects on glucose metabolism: time to unlock the controversy. Trends Endocrinol Metab. 2020;31:192–204. 10.1016/j.tem.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Minguez J, Saxena R, Bandín C, Scheer FA, Garaulet M. Late dinner impairs glucose tolerance in MTNR1B risk allele carriers: a randomized, cross-over study. Clin Nutr. 2018;37:1133–40. 10.1016/j.clnu.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z, Wang S, Gao L. Circadian rhythm, glucose metabolism and diabetic complications: the role of glucokinase and the enlightenment on future treatment. Front Physiol. 2025. 10.3389/fphys.2025.1537231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wennberg M, Gustafsson PE, Wennberg P, Hammarström A. Poor breakfast habits in adolescence predict the metabolic syndrome in adulthood. Public Health Nutr. 2015;18:122–9. 10.1017/S1368980013003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris C, Czaja K. Can circadian eating pattern adjustments reduce risk or prevent development of T2D? Nutrients. 2023;15:1762. 10.3390/nu15071762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruddick-Collins LC, Morgan PJ, Fyfe CL, Filipe JAN, Horgan GW, Westerterp KR, Johnston JD, Johnstone AM. Timing of daily calorie loading affects appetite and hunger responses without changes in energy metabolism in healthy subjects with obesity. Cell Metab. 2022;34:1472–e14856. 10.1016/j.cmet.2022.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pengpid S, Peltzer K. Skipping breakfast and its association with health risk behaviour and mental health among university students in 28 countries. Diabetes Metab Syndr Obes. 2020;13:2889–97. 10.2147/DMSO.S241670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan MSI, Paul T, Banna A, Hamiduzzaman MH, Tengan M, Kissi-Abrokwah C, Tetteh B, Hossain JK, Islam F, Brazendale MS. Skipping breakfast and its association with sociodemographic characteristics, night eating syndrome, and sleep quality among university students in Bangladesh. BMC Nutr. 2024;10:46. 10.1186/s40795-024-00860-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma X, Chen Q, Pu Y, Guo M, Jiang Z, Huang W, et al. Skipping breakfast is associated with overweight and obesity: a systematic review and meta-analysis. Obes Res Clin Pract. 2020;14:1–8. 10.1016/j.orcp.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 36.MA XM, XU Y. The association between breakfast skipping and the risk of obesity, diabetes, hypertension, or dyslipidemia—a meta-analysis from 44 trials including 65,233 cases and 381,051 controls. Diabetes. 2018;67:1356-P. 10.2337/db18-1356-P.29654212 [Google Scholar]
- 37.Wicherski J, Schlesinger S, Fischer F. Association between breakfast skipping and body weight—a systematic review and meta-analysis of observational longitudinal studies. Nutrients. 2021;13:272. 10.3390/nu13010272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Charlot A, Hutt F, Sabatier E, Zoll J. Beneficial effects of early time-restricted feeding on metabolic diseases: importance of aligning food habits with the circadian clock. Nutrients. 2021;13:1405. 10.3390/nu13051405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lyu J, Lee K, Jung S, Park YJ. Associations of meal timing and sleep duration with incidence of obesity: a prospective cohort study. J Nutr Health Aging. 2024;28:100220. 10.1016/j.jnha.2024.100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Díaz-Rizzolo DA, Santos Baez LS, Popp CJ, Borhan R, Sordi-Guth A, Manoogian ENC, Panda S, Cheng B, Laferrère B. Late eating is associated with poor glucose tolerance, independent of body weight, fat mass, energy intake and diet composition in prediabetes or early onset type 2 diabetes. Nutr Diabetes. 2024;14:90. 10.1038/s41387-024-00347-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palomar-Cros A, Andreeva VA, Fezeu LK, Julia C, Bellicha A, Kesse-Guyot E, Hercberg S, Romaguera D, Kogevinas M, Touvier M, Srour B. Dietary circadian rhythms and cardiovascular disease risk in the prospective NutriNet-Santé cohort. Nat Commun. 2023;14:7899. 10.1038/s41467-023-43444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davis R, Rogers M, Coates AM, Leung GKW, Bonham MP. The impact of meal timing on risk of weight gain and development of obesity: a review of the current evidence and opportunities for dietary intervention. Curr Diab Rep. 2022;22:147–55. 10.1007/s11892-022-01457-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gallant AR, Lundgren J, Drapeau V. The night-eating syndrome and obesity. Obes Rev. 2012;13:528–36. 10.1111/j.1467-789X.2011.00975.x. [DOI] [PubMed] [Google Scholar]
- 44.la Fleur SE, Blancas-Velazquez AS, Stenvers DJ, Kalsbeek A. Circadian influences on feeding behavior. Neuropharmacology. 2024;256:110007. 10.1016/j.neuropharm.2024.110007. [DOI] [PubMed] [Google Scholar]
- 45.Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JAJ, Ellisman MH, Panda S. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–60. 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, et al. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Health Aging. 2018;4:345–53. 10.3233/NHA-170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uti DE, Atangwho IJ, Omang WA, Alum EU, Obeten UN, Udeozor PA, et al. Cytokines as key players in obesity low grade inflammation and related complications. Obes Med. 2025;54:100585. 10.1016/j.obmed.2025.100585. [Google Scholar]
- 48.Adafer R, Messaadi W, Meddahi M, Patey A, Haderbache A, Bayen S, et al. Food timing, circadian rhythm and chrononutrition: a systematic review of time-restricted eating’s effects on human health. Nutrients. 2020;12:3770. 10.3390/nu12123770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang S, Lin X, Guan Y, Huang J. Bibliometric and visual analysis of time-restricted eating. Front Nutr. 2022. 10.3389/fnut.2022.979702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diab R, Dimachkie L, Zein O, Dakroub A, Eid AH. Intermittent fasting regulates metabolic homeostasis and improves cardiovascular health. Cell Biochem Biophys. 2024;82:1583–97. 10.1007/s12013-024-01314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bakhsh J, Salvy S-J, Vidmar AP. Intermittent fasting as a treatment for obesity in young people: a scoping review. Npj Metab Health Dis. 2024;2:39. 10.1038/s44324-024-00041-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jamshed H, Beyl RA, Della Manna DL, Yang ES, Ravussin E, Peterson CM. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients. 2019;11:1234. 10.3390/nu11061234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Santos HO, Genario R, Tinsley GM, Ribeiro P, Carteri RB, Coelho-Ravagnani C, et al. A scoping review of intermittent fasting, chronobiology, and metabolism. Am J Clin Nutr. 2022;115(4):991–1004. 10.1093/ajcn/nqab433. [DOI] [PubMed] [Google Scholar]
- 54.O’Connor SG, Boyd P, Bailey CP, Shams-White MM, Agurs-Collins T, Hall K, Reedy J, Sauter ER, Czajkowski SM. Perspective: Time-Restricted eating compared with caloric restriction: potential facilitators and barriers of Long-Term weight loss maintenance. Adv Nutr. 2021;12:325–33. 10.1093/advances/nmaa168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity. 2013;21:2504–12. 10.1002/oby.20460. [DOI] [PubMed] [Google Scholar]
- 56.Bandín C, Scheer FaJL, Luque AJ, Ávila-Gandía V, Zamora S, Madrid JA, et al. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: a randomized, crossover trial. Int J Obes (Lond). 2015;39:828–33. 10.1038/ijo.2014.182. [DOI] [PubMed] [Google Scholar]
- 57.Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31:92-e1045. 10.1016/j.cmet.2019.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee Y-C, Ordovás JM, Scheer FA. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond). 2013;37:604–11. 10.1038/ijo.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Potter GDM, Cade JE, Grant PJ, Hardie LJ. Nutrition and the circadian system. Br J Nutr. 2016;116:434–42. 10.1017/S0007114516002117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rubio-Sastre P, Scheer FAJL, Gómez-Abellán P, Madrid JA, Garaulet M. Acute melatonin administration in humans impairs glucose tolerance in both the morning and evening. Sleep. 2014;37:1715–9. 10.5665/sleep.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reutrakul S, Knutson KL. Consequences of circadian disruption on cardiometabolic health. Sleep Med Clin. 2015;10:455–68. 10.1016/j.jsmc.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mozaffarian D, Agarwal M, Aggarwal M, Alexander L, Apovian CM, Bindlish S, et al. Nutritional priorities to support GLP-1 therapy for obesity: a joint advisory from the American College of Lifestyle Medicine, the American Society for Nutrition, the Obesity Medicine Association, and the Obesity Society. Am J Clin Nutr. 2025;122:344–67. 10.1016/j.ajcnut.2025.04.023. [DOI] [PubMed] [Google Scholar]
- 63.Tricò D, Masoni MC, Baldi S, Cimbalo N, Sacchetta L, Scozzaro MT, Nesti G, Mengozzi A, Nesti L, Chiriacò M, Natali A. Early time-restricted carbohydrate consumption vs conventional dieting in type 2 diabetes: a randomised controlled trial. Diabetologia. 2024;67:263–74. 10.1007/s00125-023-06045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alum EU, Obasi DC, Abba JN, Aniokete UC, Okoroh PN, Akwari AA. Evolving paradigms in nutrition therapy for diabetes: from carbohydrate counting to precision diets. Obes Med. 2025;100622. 10.1016/j.obmed.2025.100622. [Google Scholar]
- 65.Verreijen AM, van den Helder J, Streppel MT, Rotteveel I, Heman D, van Dronkelaar C, Memelink RG, Engberink MF, Visser M, Tieland M, Weijs PJM. A higher protein intake at breakfast and lunch is associated with a higher total daily protein intake in older adults: a post-hoc cross‐sectional analysis of four randomised controlled trials. J Hum Nutr Diet. 2021;34:384–94. 10.1111/jhn.12838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sutanto CN, Wang MX, Tan D, Kim JE. Association of sleep quality and macronutrient distribution: a systematic review and meta-regression. Nutrients. 2020;12:126. 10.3390/nu12010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aoyama S, Shibata S. Time-of-day-dependent physiological responses to meal and exercise. Front Nutr. 2020. 10.3389/fnut.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.BaHammam AS, Pirzada A. Timing matters: the interplay between early mealtime, circadian rhythms, gene expression, circadian hormones, and metabolism—a narrative review. Clocks Sleep. 2023;5:507–35. 10.3390/clockssleep5030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Masi D, Spoltore ME, Rossetti R, Watanabe M, Tozzi R, Caputi A, et al. The influence of ketone bodies on circadian processes regarding appetite, sleep and hormone release: a systematic review of the literature. Nutrients. 2022;14:1410. 10.3390/nu14071410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Konstantinidou V, Jamshed H. Editorial: Chrononutrition and health. Front Nutr. 2024. 10.3389/fnut.2024.1516940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yanagi S, Sato T, Kangawa K, Nakazato M. The homeostatic force of Ghrelin. Cell Metab. 2018;27:786–804. 10.1016/j.cmet.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 72.Picó C, Palou M, Pomar CA, Rodríguez AM, Palou A. Leptin as a key regulator of the adipose organ. Rev Endocr Metab Disord. 2022;23:13–30. 10.1007/s11154-021-09687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vijayashankar U, Ramashetty R, Rajeshekara M, Vishwanath N, Yadav AK, Prashant A, Lokeshwaraiah R. Leptin and Ghrelin dynamics: unraveling their influence on food intake, energy balance, and the pathophysiology of type 2 diabetes mellitus. J Diabetes Metab Disord. 2024;23:427–40. 10.1007/s40200-024-01418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Andreadi A, Andreadi S, Todaro F, Ippoliti L, Bellia A, Magrini A, et al. Modified cortisol circadian rhythm: the hidden toll of night-shift work. Int J Mol Sci. 2025;26:2090. 10.3390/ijms26052090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Albreiki MS, Middleton B, Hampton SM. The effect of melatonin on glucose tolerance, insulin sensitivity and lipid profiles after a late evening meal in healthy young males. J Pineal Res. 2021;71:e12770. 10.1111/jpi.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Papatriantafyllou E, Efthymiou D, Zoumbaneas E, Popescu CA, Vassilopoulou E. Sleep deprivation: effects on weight loss and weight loss maintenance. Nutrients. 2022;14:1549. 10.3390/nu14081549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arora T, Choudhury S, Taheri S. The relationships among sleep, nutrition, and obesity. Curr Sleep Med Rep. 2015;1:218–25. 10.1007/s40675-015-0030-z. [Google Scholar]
- 78.Alum EU. Metabolic memory in obesity: can early-life interventions reverse lifelong risks? Obes Med. 2025;55:100610. 10.1016/j.obmed.2025.100610. [Google Scholar]
- 79.Schettini MAS, Passos RF, do N, Koike BDV. Shift work and metabolic syndrome updates: a systematic review. Sleep Sci. 2023;16:237–47. 10.1055/s-0043-1770798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gutierrez Lopez DE, Lashinger LM, Weinstock GM, Bray MS. Circadian rhythms and the gut microbiome synchronize the host’s metabolic response to diet. Cell Metab. 2021;33:873–87. 10.1016/j.cmet.2021.03.015. [DOI] [PubMed] [Google Scholar]
- 81.Minabe K, Shimura A, Sugiura K, Hino H, Akatsuka Y, Seto T, Yanai M, Masuya J, Tamada Y, Inoue T. Association between social jetlag and weight and fat reduction in dieting. Sleep Biol Rhythms. 2024;22:513–21. 10.1007/s41105-024-00539-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mota MC, Silva CM, Balieiro LCT, Fahmy WM, Crispim CA. Social jetlag and metabolic control in non-communicable chronic diseases: a study addressing different obesity statuses. Sci Rep. 2017;7:6358. 10.1038/s41598-017-06723-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Islam Z, Akter S, Kochi T, Hu H, Eguchi M, Yamaguchi M, Kuwahara K, Kabe I, Mizoue T. Association of social jetlag with metabolic syndrome among Japanese working population: the Furukawa nutrition and health study. Sleep Med. 2018;51:53–8. 10.1016/j.sleep.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 84.Bouman EJ, Beulens JWJ, den Braver NR, Blom MT, Remmelzwaal S, Elders PJM, Rutters F. Social jet lag and (changes in) glycemic and metabolic control in people with type 2 diabetes. Obesity. 2023;31:945–54. 10.1002/oby.23730. [DOI] [PubMed] [Google Scholar]
- 85.Qian J, Scheer FAJL. Circadian system and glucose metabolism: implications for physiology and disease. Trends Endocrinol Metab. 2016;27:282–93. 10.1016/j.tem.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.LeBlanc KE, Baer-Sinnott S, Lancaster KJ, Campos H, Lau KHK, Tucker KL, et al. Perspective: beyond the Mediterranean diet—exploring Latin American, Asian, and African heritage diets as cultural models of healthy eating. Adv Nutr. 2024;15:100221. 10.1016/j.advnut.2024.100221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garaulet M, Gómez-Abellán P. Timing of food intake and obesity: a novel association. Physiol Behav. 2014;134:44–50. 10.1016/j.physbeh.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 88.Pot GK, Hardy R, Stephen AM. Irregular consumption of energy intake in meals is associated with a higher cardiometabolic risk in adults of a British birth cohort. Int J Obes (Lond). 2014;38:1518–24. 10.1038/ijo.2014.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rosa I, Lages M, Grilo C, Barros R, Guarino MP. Mhealth applications to monitor lifestyle behaviors and circadian rhythm in clinical settings: current perspective and future directions. Front Public Health. 2022;10:862065. 10.3389/fpubh.2022.862065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ezenwaji CO, Alum EU, Ugwu OP-C. The role of digital health in pandemic preparedness and response: Securing global health? Global Health Action. 2024;17:2419694. 10.1080/16549716.2024.2419694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chellappa SL, Gao L, Qian J, Vujovic N, Li P, Hu K, Scheer FAJL. Daytime eating during simulated night work mitigates changes in cardiovascular risk factors: secondary analyses of a randomized controlled trial. Nat Commun. 2025;16:3186. 10.1038/s41467-025-57846-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peters B, Vahlhaus J, Pivovarova-Ramich O. Meal timing and its role in obesity and associated diseases. Front Endocrinol (Lausanne). 2024;15:1359772. 10.3389/fendo.2024.1359772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Varma P, Shen L, Postnova S, King K, Howard ME, Aidman E, Rajaratnam SWM, Sletten TL. Understanding sleep health challenges of defence shift workers to design a digital, sleep and circadian management tool. Sci Rep. 2025;15:10483. 10.1038/s41598-025-93597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hood S, Amir S. The aging clock: circadian rhythms and later life. J Clin Invest. 2017;127:437–46. 10.1172/JCI90328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Palmese F, Druda Y, Del Toro R, Bedogni G, Domenicali M, Silvani A. The role of the circadian timing system in sarcopenia in old age: a scoping review. Eur Geriatr Med. 2025;16:447–60. 10.1007/s41999-024-01129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lowe DA, Wu N, Rohdin-Bibby L, Moore AH, Kelly N, Liu YE, et al. Effects of time-restricted eating on weight loss and other metabolic parameters in women and men with overweight and obesity: the TREAT randomized clinical trial. JAMA Intern Med. 2020;180:1491–9. 10.1001/jamainternmed.2020.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Abbott SM, Malkani RG, Zee PC. Circadian disruption and human health: a bidirectional relationship. Eur J Neurosci. 2020;51:567–83. 10.1111/ejn.14298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mezhnina V, Ebeigbe OP, Poe A, Kondratov RV. Circadian control of mitochondria in reactive oxygen species homeostasis. Antioxid Redox Signal. 2022;37:647–63. 10.1089/ars.2021.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sasidharan Pillai S, Gagnon CA, Foster C, Ashraf AP. Exploring the gut microbiota: key insights into its role in obesity, metabolic syndrome, and type 2 diabetes. J Clin Endocrinol Metab. 2024;109:2709–19. 10.1210/clinem/dgae499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ronteltap A, Bukman AJ, Nagelhout GE, Hermans RCJ, Hosper K, Haveman-Nies A, Lupker R, Bolman CAW. Digital health interventions to improve eating behaviour of people with a lower socioeconomic position: a scoping review of behaviour change techniques. BMC Nutr. 2022;8:145. 10.1186/s40795-022-00635-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated during this study are included in the manuscript.