Abstract
The misfolding of transthyretin (TTR), including rate-limiting tetramer dissociation and partial monomer denaturation, is sufficient for TTR misassembly into amyloid and other abnormal quaternary structures associated with three amyloid diseases: senile systemic amyloidosis, familial amyloid polyneuropathy, and familial amyloid cardiomyopathy. Small molecules can bind to one or both of the unoccupied TTR thyroid hormone-binding sites, stabilizing the native tetramer more than the dissociative transition state, thereby raising the kinetic barrier for tetramer dissociation. Herein we demonstrate that genistein, the major isoflavone natural product in soy, works in this fashion and is an excellent inhibitor of transthyretin tetramer dissociation and amyloidogenesis, reducing acid-mediated fibril formation to <10% of that exhibited by TTR alone. Genistein also inhibits the amyloidogenesis of the most common familial amyloid polyneuropathy and familial amyloid cardiomyopathy mutations in TTR: V30M and V122I, respectively. Genistein additionally inhibits tetramer dissociation under physiological conditions thought to lead to slow amyloidogenesis in humans. Furthermore, this natural product exhibits highly selective binding to TTR in plasma over all of the other plasma proteins. Isothermal titration calorimetry shows that genistein binds to TTR with negative cooperativity (Kd1 = 40 nM, Kd2 = 1.4 μM). The benefits of using a nutraceutical such as genistein to treat orphan diseases such as the TTR amyloidoses include known oral bioavailability and safety data. It is conceivable that some patients could benefit from simply increasing their intake of soy products or supplements.
Keywords: amyloidogenesis inhibitor, kinetic stabilization
For many years nutritionists and dieticians have noted the health benefits of a soy-based diet, citing the much lower incidence of cancer, including breast cancer, in Asian countries (1–4). The isoflavone genistein (compound 1 in Fig. 1), found in various soy foods at concentrations of 1.9–229 μg/g, is the component of soy implicated in cancer chemoprevention (5). An additional 71–968 μg/g of genistein is present as its O-glucoside conjugate, genistin (2), which is rapidly deglycosylated by intestinal bacteria in vivo. Genistein is being evaluated in preliminary trials for treatment of breast, prostate, and uterine cancers (6, 7), as well as for osteoporosis (8), cardiovascular disease (9), and treatment of menopausal symptoms (10). Toxicity studies reveal that this isoflavone does not appear to cause adverse health effects, even at the relatively high concentrations used (11–13). The isoflavone daidzein (3), lacking the hydroxyl group at the 5 position of genistein, is also found in soy foods, but no chemoprotective effects have been attributed to it.
Fig. 1.
Line drawings depicting the structures of genistein (1), genistin (2), daidzein (3), daidzin (4), and apigenin (5) and the numbering of the isoflavone ring system.
Transthyretin (TTR) is a tetrameric protein composed of identical 127-aa β-sheet sandwich subunits (14, 15). TTR functions to transport holo-retinol-binding protein and thyroxine (T4) in the blood and cerebrospinal fluid (16, 17). Under denaturing conditions, the TTR tetramer dissociates and the monomers partially unfold and misassemble into amyloid fibrils and amorphous aggregates (18–22). This process also occurs very slowly under physiological conditions (23–25). Senile systemic amyloidosis (26, 27) is characterized by the deposition of WT TTR in the heart and peripheral nerves, whereas the deposition of one of >100 different TTR variants is associated with a group of diseases collectively known as the familial amyloidoses. The V30M mutation is the most common familial amyloid polyneuropathy variant and has been found in patients in Japan, Portugal, and Sweden (28, 29). Approximately 1 million African Americans are at significant risk for congestive heart failure due to the familial amyloid cardiomyopathy variant, V122I TTR, having high penetrance (30). In addition, a subset of TTR variants has recently been shown to exhibit CNS-selective amyloidosis.
V30M familial amyloid polyneuropathy is effectively treated by liver transplantation (31, 32), which mediates the replacement of the V30M allele by a WT allele in the organ that supplies tissues except the brain and eyes with TTR. However, emerging clinical data suggest that this procedure is substantially less effective against other familial TTR mutations for reasons that are unclear. Furthermore, transplantation is not an option for treating senile systemic amyloidosis, which results from deposition of WT TTR (33, 34). Nor would this approach be useful for ameliorating CNS-selective amyloidosis because the TTR deposited in these tissues is synthesized in the choroid plexus. Therefore, a general chemotherapeutic option would be highly desirable as a treatment strategy.
TTR has two identical funnel-shaped thyroxine-binding sites located at the dimer–dimer interface, which can be interconverted by a C2 operation about the x or y axis oriented perpendicular to the crystallographic twofold axis (z axis; see Fig. 2) (35). Typically, <1% of TTR in the plasma and cerebrospinal fluid is bound to thyroxine, allowing us to target these sites with other small aromatic molecules to prevent amyloidogenesis (36). By using both focused screening and structure-based design, our laboratory has previously reported several classes of compounds that are capable of inhibiting TTR fibril formation by binding to the thyroxine sites (37–47). Good inhibitors bind with high affinity, dissociate slowly, and exhibit high binding selectivity to TTR in the blood. These molecules exert their effects through kinetic stabilization mediated by preferential binding to the native state over the dissociative transition state (48). Kinetic stabilization of the native state is the same mechanism operating in compound heterozygotes, where incorporation of T119M trans-suppressor subunits into tetramers otherwise composed of V30M subunits raises the dissociation activation barrier, thereby ameliorating disease (48, 49). Therefore, it is reasonable to be optimistic that small molecule kinetic stabilization would prevent the majority of TTR amyloidoses, with the caveat that influencing CNS-selective amyloidosis would require blood–brain barrier penetration. Given the orphan disease status of the TTR amyloidoses, it would greatly accelerate clinical trials if one could find a highly efficacious natural product with an established safety profile in humans.
Fig. 2.
Schematic representation of the tetrameric structure of TTR depicting the two thyroxine-binding sites. The two binding sites are interconverted by two C2 axes perpendicular to the crystallographic twofold axis. Each binding site, filled with thyroxine, has an inner and outer binding pocket.
We have tested the natural product genistein and several structurally related analogs for their ability to inhibit TTR amyloid fibril formation in vitro. Genistein appears to be an exceptional inhibitor of WT TTR amyloidogenesis. Moreover, this compound exhibits highly selective binding to TTR in plasma over all other possible protein targets. Genistein also inhibits amyloidogenesis of the most common disease-associated variants: V30M and V122I. The benefits of using such a nutraceutical are many; it is possible that some patients may benefit simply from increasing their intake of soy products or adding a soy-based supplement to their diets, but further research is needed. The wealth of toxicity information on genistein suggests that it is safe for human consumption, even at the high concentrations used for cancer trials (11–13). This compound, however, is known to target multiple pathways (7, 50) and, as such, must be used cautiously.
Materials and Methods
Genistein, daidzein, and apigenin were purchased from Aldrich. Genistin was purchased from Calbiochem and used as provided. The purity of these compounds was established by HPLC and high-resolution MS.
Protein Expression and Purification. WT, V122I, V30M, and dual-FLAG-tagged TTR (FT)4 were expressed and purified from Escherichia coli as described in ref. 51.
Stagnant Acid-Mediated TTR Aggregation Assay. Stagnant aggregation assays were performed as described in ref. 23. A 0.495-ml sample of TTR [7.2 μM tetramer (0.4 mg/ml) in 10 mM sodium phosphate/100 mM KCl/1 mM EDTA, pH 7] was incubated with 5 μl of isoflavone or flavone inhibitor in DMSO (0.72, 1.44, or 7.2 mM). After 30 min, the samples were diluted with 0.5 ml of 200 mM sodium acetate buffer (pH 4.2, final pH 4.4 for WT and V122I and pH 4.8, final pH 5 for V30M) containing 100 mM KCl and 1 mM EDTA. Samples were briefly vortexed and then further incubated at 37°C for 72 h without stirring. The extent of aggregation was probed by turbidity measurements at 350 and 400 nm on an HP 8453 UV–visible spectrometer. Single time-point samples (72 h) were vortexed immediately before the measurement.
Urea-Mediated TTR Tetramer Dissociation Kinetics Measured by CD. TTR (400 μl of 4.5 μM tetramer; 0.25 mg/ml) was preincubated with genistein at either 4.5 or 9.0 μM for 18 h at 25°C. Urea (10 M, 600 μl) in 50 mM sodium phosphate buffer (pH 7) containing 100 mM KCl, 1 mM EDTA, and 1 mM DTT was added to the samples immediately before the first measurement [1.0 ml total volume, 6.0 M urea, 0.1 mg/ml TTR (1.8 μM tetramer) final concentration]. CD spectra were recorded as a function of time up to 120 h (25°C) by using a wavelength scan from 220 to 214 nm, sampling every 0.5 nm. The signal from 218 to 215 nm was averaged and plotted to determine the fraction of TTR tetramer that was dissociated and unfolded at each time point.
TTR Tetramer Dissociation Kinetics at Neutral pH Followed by Subunit Exchange. TTR (500 μl, 1.8 μM tetramer) and dual-FLAG-tagged TTR (FT)4 (500 μl, 1.8 μM tetramer) were mixed and incubated with 0, 3.6, or 7.2 μM genistein at 23°C to evaluate the rate of tetramer dissociation under physiological conditions as described in ref. 52. At the desired time points, 50-μl aliquots were loaded onto a Mono Q PC 1.6/5 anion-exchange column using an Amersham Pharmacia SMART system equilibrated in 24% solution A (25 mM Tris, pH 8/1 mM EDTA) and 76% solution B (solution A + 1 M NaCl). A linear elution gradient from 24% A to 42% A over 45 min produced chromatograms with five distinct tetramer peaks corresponding to (TTR)4, (TTR)3(FT)1, (TTR)2(FT)2, (TTR)1(FT)3, and (FT)4. Percentage exchange was determined by dividing the relative proportion of a peak at each time point by its statistical endpoint, with the equilibrium distribution ratio being 1:4:6:4:1 for peaks 1–5, respectively (i.e., peak 3 will account for 6/16 (37.5%) of the total protein at equilibrium).
TTR Antibody Purification and Conjugation to Sepharose. Antibodies, raised as described in ref. 53, were purified by passage of rabbit serum over a recombinant staphylococcal protein A column. The column was washed with 5 column volumes of 50 mM sodium phosphate buffer (pH 7.2), and the antibodies were eluted with 5 column volumes of 100 mM sodium citrate buffer (pH 3). Each 5-ml elution fraction was neutralized with 1 ml of 1 M Tris·HCl buffer (pH 9). The fractions were then dialyzed against 100 mM sodium bicarbonate, pH 8.2. The concentrated protein (7 mg/ml) was then coupled to cyanogen bromide-activated Sepharose as described in ref. 53. The Sepharose gel (1 g of gel per 35 mg of protein) was first washed in a filter funnel with 1,400 ml of 1 mM HCl for 15 min. The coupling buffer (100 mM sodium bicarbonate/500 mM NaCl, pH 8.3) and the antibody were added to the washed gel (5 ml coupling buffer/35 mg of antibody per g of gel). The gel was rotated at room temperature for 1 h, followed by centrifugation at 3,000 rpm for 1 min in an Eppendorf 5415C centrifuge. The gel was transferred to 100 mM Tris·HCl buffer (pH 8) and rotated at room temperature for 2 h. The gel was washed with 100 mM sodium acetate buffer (pH 4)/500 mM NaCl and 100 mM Tris·HCl buffer (pH 8)/500 mM NaCl for two cycles. The gel was washed twice with TSA (10 mM Tris·HCl/140 mM NaCl/0.025% sodium azide, pH 8) and stored as a 1:1 slurry in TSA.
Plasma Selectivity Binding of Genistein and Daidzein to TTR. The binding stoichiometry of genistein and daidzein to TTR in blood plasma was determined by an antibody capture/HPLC method (53). A sample of 7.5 μl of a 1.44 mM DMSO stock solution of potential inhibitor was added to a 1.5 ml Eppendorf tube containing 1 ml of human blood plasma. The mixture was incubated at 37°C for 18 h. A 1:1 gel/TSA slurry (125 μl) of quenched Sepharose was added and the resulting slurry was rocked for 1 h at 4°C. The mixture was centrifuged (16,000 × g) and the supernatant was divided into two 400-μl aliquots. To each aliquot was added 200 μl of a 1:1 gel/TSA slurry of the anti-TTR antibody-conjugated Sepharose (see above). These mixtures were rocked slowly for 20 min at 4°C, followed by centrifugation (16,000 × g) and removal of the supernatant. The gel pellet was washed with 1 ml of TSA with 0.05% saponin (three times for 10 min each) at 4°C, followed by two 1-ml washes with TSA (10 min each). The samples were centrifuged (16,000 × g) after the final wash, and 155 μl of 100 mM triethylamine (pH 11.5) was added to the resultant pellet to elute the TTR and bound small molecules from the antibody. The high-pH mixture was rocked at 4°C for 30 min and then centrifuged (16,000 × g). The supernatant (145 μl) containing TTR and inhibitor was removed and analyzed by reversed-phase HPLC. The resulting solution (135 μl) was injected with a Waters 717Plus autosampler onto a Keystone Scientific (Bellefonte, PA) 3-cm C18 reversed-phase column at 100% solution C. A 20–100% linear gradient of solution D over 9 min was used to elute both TTR and inhibitor. Solution C is composed of 94.8% water, 5% acetonitrile, and 0.2% trifluoroacetic acid. Solution D contains 94.8% acetonitrile, 5% water, and 0.2% trifluoroacetic acid. Detection at 280 nm was accomplished with a Waters 486 tunable absorbance detector. The integrated peaks of the small molecule and TTR were compared to calibration curves prepared from known amounts of small molecule and TTR.
Isothermal Titration Calorimetry. The dissociation constants characterizing the binding of genistein to WT TTR were determined by using a Microcal (Amherst, MA) isothermal titration calorimeter. A solution of genistein (final concentration 432 μM in 25 mM Tris, pH 8/100 mM KCl/1 mM EDTA/10% EtOH,) was prepared and titrated into an isothermal titration calorimetry cell containing WT TTR (12 μM in 25 mM Tris, pH 8/100 mM KCl/1 mM EDTA/10% EtOH). For all runs, a small preliminary injection was followed by identical injections (2–5 μl) up to a ligand-to-protein molar ratio of at least 4:1. The data were fit by a nonlinear least-squares approach to either an identical binding sites model or a sequential interacting binding sites model (51) by using Microsoft excel (Redmond, WA) with the solver plugin.
Results
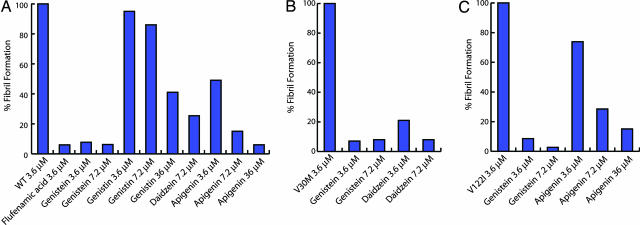
Fibril Formation Assays. Genistein (1; Fig. 1), genistin (2), daidzein (3), and apigenin (5) were tested as potential inhibitors of WT TTR amyloidogenesis, using a turbidity assay described previously and validated by a thioflavin T fluorescence assay (23, 54). These prominent components of soy were evaluated because a soy extract exhibited activity in a screen for natural product inhibitors of TTR amyloidosis (N.S.G., unpublished results). Genistein was also tested as an amyloidogenesis inhibitor of the most common familial amyloid polyneuropathy and familial amyloid cardiomyopathy TTR mutations, V30M and V122I, respectively. Aggregate formation is reported relative to WT or mutant TTR homotetramer, where amount of aggregation in the absence of inhibitor is assigned to be 100% [≈50% chemical yield at 3.6 μM (21)]. Hence 5% aggregate formation in the presence of a given inhibitor corresponds to 95% inhibition. Genistein essentially prevented acid-mediated aggregation (2–9% fibrils) from WT, V30M, and V122I TTR (3.6 μM) at both concentrations of inhibitor tested (3.6 and 7.2 μM) (Fig. 3). Daidzein and apigenin were less effective inhibitors of WT aggregate formation, allowing ≈20% and ≈28% aggregation, respectively, when administered at a concentration (7.2 μM) twice that of TTR (3.6 μM). The glucoside genistin was a very poor inhibitor, displaying 41% WT TTR aggregate formation at a concentration an order of magnitude higher (36 μM) than that of TTR.
Fig. 3.
Partial acid denaturation-mediated aggregation of WT (A), V30M (B), and V122I (C) TTR. Blue bars represent data from an aggregate formation assay wherein tetrameric TTR (3.6 μM) is preincubated with inhibitor (3.6, 7.2, or 36 μM) for 30 min before lowering the pH to 4.4 (72 h). The y axis in each bar graph (optical density at 350 nm) represents aggregate formation relative to TTR (WT or variant, 3.6 μM) without inhibitor assigned as 100%. Hence 5% aggregate formation equates to 95% inhibition. The absolute turbidity OD350 values for the uninhibited reactions are WT, 1.25; V30M, 1.36; and V122I, 1.10.
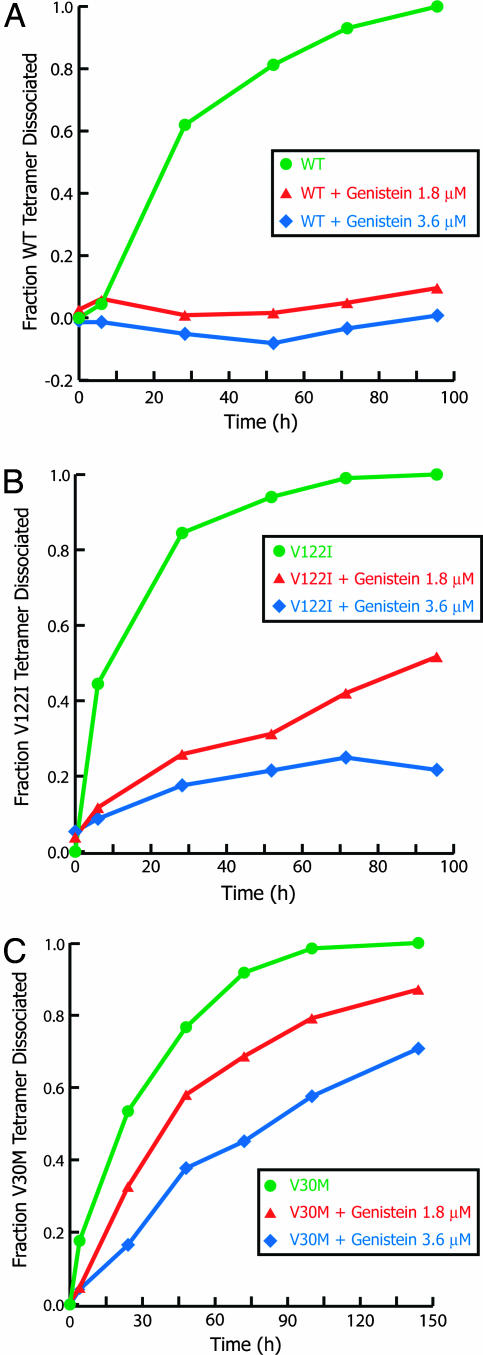
Rate of Tetramer Dissociation as a Function of Genistein Concentration in Denaturant. Genistein was further tested for its ability to kinetically stabilize tetrameric TTR against urea-induced dissociation. Because dissociation of the tetramer is required for urea-induced monomer denaturation, it is possible to monitor rate-limiting tetramer dissociation by linking this process to fast monomer unfolding in a posttransition urea concentration (6 M) that renders the process irreversible. The rate and extent of TTR tetramer (1.8 μM) dissociation at two small molecule concentrations (1.8 and 3.6 μM) was monitored by far-UV CD spectroscopy in 6 M urea. Genistein exerts its most dramatic effect on the amplitude of WT TTR tetramer dissociation (Fig. 4A). At equimolar amounts of genistein and WT TTR (1.8 μM), only 10% of the protein dissociates and unfolds after 120 h, implying that the remainder is stabilized as a consequence of small molecule binding. This result compares to 53% of dissociation for V122I (Fig. 4B, triangles) and 87% for V30M (Fig. 4C, triangles), dissociating under identical conditions. When the inhibitor concentration (3.6 μM) is twice that of TTR (1.8 μM), only 1% of WT TTR (Fig. 4A, diamonds), 18% of V122I (Fig. 4B, diamonds), and 70% of V30M (Fig. 4C, diamonds) dissociates over the same time period. These results are consistent with small-molecule-binding-imposed kinetic stabilization of the TTR tetramer.
Fig. 4.
The rate of urea-mediated (6 M) tetramer dissociation for WT (A) (green circles), V122I (B), and V30M (C) TTR in the absence of small molecules. TTR dissociation is slowed dramatically when WT and the variants are preincubated with genistein. Far-UV CD ellipticity integrated over 214–218 nm at two concentrations of genistein (1.8 μM, ▴; 3.6 μM, ♦) was compared with that of TTR (WT or variant, 1.8 μM) without genistein to determine the fraction of TTR that dissociated and rapidly unfolded at each time point. As a reference, WT TTR tetramer dissociation occurs with a first-order rate constant of 0.033 h-1 under these conditions.
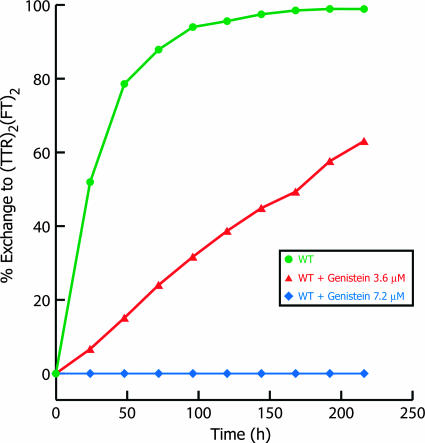
Rate of Tetramer Dissociation as a Function of Genistein Concentration Under Conditions Simulating Physiological Conditions. The ability of genistein to kinetically stabilize tetrameric TTR under nondenaturing conditions at neutral pH was evaluated by using a subunit exchange assay described in ref. 55. Tetramer dissociation is rate limiting for subunit exchange and thus can be used to examine tetramer kinetic stabilization by small molecules (52). At equimolar genistein (3.6 μM genistein and TTR), TTR exhibits a greatly reduced exchange rate and a reduced extent of exchange (50% exchange reached after 168 h with equimolar genistein, compared with 24 h without genistein; Fig. 5, triangles). At a genistein concentration (7.2 μM) twice the TTR concentration (3.6 μM), no exchange and thus no dissociation is observed over 216 h (Fig. 5, diamonds). These results demonstrate that genistein is able to kinetically stabilize TTR tetramers under neutral, nondenaturing conditions that more closely resemble physiological conditions.
Fig. 5.
Rate of WT TTR (1.8 μM tetramer) homotetramer subunit exchange with subunits from homotetramers of FT (1.8 μM tetramer) can be used to follow the rate of tetramer dissociation, because dissociation is rate-limiting for subunit exchange under physiological conditions. Subunit exchange kinetics decrease substantially at an equimolar concentration of genistein (3.6 μM, ▴), whereas, at twice the TTR concentration, genistein completely eliminates tetramer dissociation (7.2 μM, ♦). Percentage exchange is calculated by dividing the concentration of (TTR)2(FT)2 at each time point by its equilibrium concentration in the absence of inhibitor.
Plasma Selectivity of Genistein and Daidzein. To test whether genistein and daidzein could bind selectively to TTR over all other proteins in the blood plasma, these two compounds were incubated with plasma and their binding stoichiometry to TTR was determined. The two isoflavones were separately incubated with human plasma at a concentration of 10.8 μM (typical TTR concentration in human plasma is ≈5 μM). TTR was captured with a resin-bound anti-TTR antibody and subjected to five wash steps. After high-pH release of TTR and any bound small molecule from the antibody, the stoichiometry of inhibitor binding to TTR was evaluated by reversed-phase HPLC. A maximum of 2.0 eq of inhibitor may be bound per TTR tetramer. It is established that wash-associated losses lower the observed stoichiometry, and therefore the measured stoichiometries should be considered lower limits (Y. Sekijima and J.W.K., unpublished data). An analysis of four separate experiments reveals a plasma selectivity for genistein of 1.45 eq per tetramer, implying that the dissociation constants of the ligand are very low, and genistein is thus a high-affinity ligand. Daidzein, on the other hand, displays a binding stoichiometry of 0.75.
Determination of Binding Constants of Genistein to WT TTR. Isothermal titration calorimetry was used to determine the dissociation constants of the binding of genistein to WT TTR at pH 8 (25°C). Integration of the thermogram after subtraction of blanks yielded a binding isotherm that fit equally well to a model of two sequential interacting binding sites with negative cooperativity or two identical noninteracting sites. The fit to sequential binding sites yielded dissociation constants of Kd1 = 40 ± 25 nM and Kd2 = 1,400 ± 170 nM. Fitting the data to identical binding sites gave Kd1 = Kd2 = 845 ± 45 nM with an occupancy of 1.92 ± 0.07. The inhibition efficacy strongly suggests negatively cooperative binding (Kd1 = 40 ± 25 nM and Kd2 = 1,400 ± 170 nM); see below.
Discussion
Because it is not established how and where amyloidogenesis occurs in humans, we have evaluated genistein under a variety of conditions to demonstrate kinetic stabilization independent of conditions used. Genistein is an excellent acid-mediated TTR amyloidogenesis inhibitor. This nutraceutical (3.6 μM or 7.2 μM) substantially inhibits WT, V30M, and V122I TTR amyloidogenesis (Fig. 3 A, B, and C, respectively) to <10% of that exhibited by unliganded TTR (3.6 μM TTR). In addition, genistein dramatically slows the rate of WT and V122I TTR tetramer dissociation in concentrated urea solutions (Fig. 4), demonstrating small-molecule-mediated kinetic stabilization of the tetramer. The lesser effect observed with V30M does not necessarily imply that genistein would be insufficient to treat V30M disease, because these experiments utilize urea solutions that are unlikely to accurately simulate the physiological conditions under which amyloidogenesis occurs in humans; rather they are used to demonstrate kinetic stability. Because it is thought that TTR dissociation and subsequent amyloidogenesis occur slowly at neutral pH, we demonstrate herein, by using the recently described subunit exchange inhibition method (52), that genistein can impose kinetic stabilization on TTR under physiological conditions (Fig. 5).
Kinetic stabilization of the TTR tetramer results from selective stabilization of the native state over the dissociative transition states. Kinetic stabilization of V30M-containing TTR tetramers by inclusion of T119M subunits is sufficient to ameliorate TTR amyloidosis, suggesting that genistein-mediated kinetic stabilization of TTR should be effective at preventing disease in humans. Kinetic stabilization of the TTR tetramer is the most conservative strategy because it remains unclear what species on the TTR amyloidogenesis pathway induces toxicity.
Evaluation of isothermal titration calorimetry data reveals that the binding constants of genistein for WT TTR at pH 8 (25°C) are either Kd1 = 40 nM, Kd2 = 1,400 nM, or Kd1 = Kd2 = 845 nM. Given the strong aggregation inhibition observed at equal concentrations of genistein and TTR, it appears likely that genistein binds with negative cooperativity because Kd1 = 40 nM and Kd2 = 1,400 nM affords predominantly TTR·I, consistent with the recent discovery that occupancy of only one ligand-binding site is sufficient to impose kinetic stabilization on the entire TTR tetramer (47, 51). The efficacy at the low concentration would not be expected if Kd1 = Kd2 = 845 nM because unliganded amyloidogenic TTR would be the major species.
The hydroxyl groups in positions 5 and 7 of genistein seem to be important for aggregation inhibition. Daidzein, lacking the 5-OH, has an ≈4-fold decrease in aggregation inhibition potency when administered at a concentration (7.2 μM) twice that of TTR (3.6 μM). Masking the hydroxyl group at position 7 with a glucose moiety (genistin) leads to a dramatic loss of activity (41% aggregate formation) even at very high inhibitor concentrations (36 μM genistin and 3.6 μM TTR). The position of the p-hydroxyphenyl substituent also appears to be important. Moving this substructure from position 2 of the isoflavone (genistein) to position 1 (apigenin), results in a 2-fold decrease in WT TTR aggregation inhibition at pH 4.4.
We have reported the efficacy of diflunisal, a nonsteroidal anti-inflammatory drug, for the inhibition of TTR amyloidogenesis (56). Although this compound shows promise in a normal human subjects oral dosing study (Y. Sekijima and J.W.K., unpublished results), it may be problematic for the treatment of V122I familial amyloid cardiomyopathy owing to compromised renal blood flow in the African American population, which suffers from a much higher incidence of kidney disease and failure (U.S. Renal Data System database, www.usrds.org). Treatment with nonsteroidal anti-inflammatory drugs will likely exacerbate this risk because they inhibit the synthesis of prostaglandins, which help to maintain blood flow to the kidneys. Genistein may be a better V122I amyloidosis inhibitor because it has not been shown to have any adverse effects on kidney function and is more active and selective than diflunisal.
A significant, but not insurmountable, issue is that the oral bioavailabilities of genistein and genistin are modest, with in vivo plasma concentrations of genistein ≈0.1–8 μM at a dose of 16 mg/kg of body weight (12, 13, 57). Liu and Hu's (58) study using Caco-2 cells and perfused rat intestinal models shows that genistein is efficiently absorbed into the intestine, but extensive first-pass metabolism results in formation of 7-OH-glucuronic acid as the major metabolite. The permeance of genistin was ≈5-fold lower than its corresponding aglycone. The half-life of genistein in plasma was determined to be 3.2 h for men and 3.8 h for women. These appealing pharmacokinetics suggest that a slow-release formula could be useful (12, 13).
Soy products, and genistein in particular, have been reported to have antitumor effects through the inhibition of protein tyrosine kinase pathways leading to gene expression modification of many proteins, including VEGF. These expression changes have been shown to arrest cell growth and proliferation, angiogenesis, and the cell cycle at G2/M (50). The interaction of genistein with tyrosine kinases and their influence on numerous biological pathways poses a concern for long-term therapy. These concerns are tempered both by epidemiological data, suggesting that diets high in soy have numerous positive effects, and by numerous short-term high-dose studies evaluating the toxicity of genistein.
Summary
Genistein is an excellent acid-mediated TTR amyloidogenesis inhibitor in vitro; it kinetically stabilizes TTR under both native and denaturing conditions in vitro; it exhibits excellent binding selectivity in plasma ex vivo and appears worthy of further preclinical studies.
Acknowledgments
We thank the National Institutes of Health for Grant DK46335 and the Skaggs Institute of Chemical Biology and the Lita Annenberg Hazen Foundation for generous financial support. N.S.G. thanks the National Institutes of Health for National Research Service Award Grant DK060304-03. T.R.F. thanks the David and Ursula Fairchild Graduate Student Fellowship for generous support.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TTR, transthyretin; FT, dual-FLAG-tagged TTR.
References
- 1.Setchell, K. D., Borriello, S. P., Hulme, P., Kirk, D. N. & Axelson, M. (1984) Am. J. Clin. Nutr. 40, 569-578. [DOI] [PubMed] [Google Scholar]
- 2.Lee, H. P., Gourley, L., Duffy, S. W., Esteve, J., Lee, J. & Day, N. E. (1991) Lancet 337, 1197-1200. [DOI] [PubMed] [Google Scholar]
- 3.Rose, D. P. (1992) Nutrition 8, 47-51. [PubMed] [Google Scholar]
- 4.Wu, A. H., Ziegler, R. G., Horn-Ross, P. L., Nomura, A. M., West, D. W., Kolonel, L. N., Rosenthal, J. F., Hoover, R. N. & Pike, M. C. (1996) Cancer Epidemiol. Biomarkers Prev. 5, 901-906. [PubMed] [Google Scholar]
- 5.Fukutake, M., Takahashi, M., Ishida, K., Kawamura, H., Sugimura, T. & Wakabayashi, K. (1996) Food Chem. Toxicol. 34, 457-461. [DOI] [PubMed] [Google Scholar]
- 6.Sarkar, F. H. & Li, Y. (2002) Cancer Metastasis Rev. 21, 265-280. [DOI] [PubMed] [Google Scholar]
- 7.Polkowski, K. & Mazurek, A. P. (2000) Acta Pol. Pharm. 57, 135-155. [PubMed] [Google Scholar]
- 8.Potter, S. M., Baum, J. A., Teng, H., Stillman, R. J., Shay, N. F. & Erdman, J. W., Jr. (1998) Am. J. Clin. Nutr. 68, 1375S-1379S. [DOI] [PubMed] [Google Scholar]
- 9.Cai, Q. & Wei, H. (1996) Nutr. Cancer 25, 1-7. [DOI] [PubMed] [Google Scholar]
- 10.Zava, D. T. & Duwe, G. (1997) Nutr. Cancer 27, 31-40. [DOI] [PubMed] [Google Scholar]
- 11.Okazaki, K., Okazaki, S., Nakamura, H., Kitamura, Y., Hatayama, K., Wakabayashi, S., Tsuda, T., Katsumata, T., Nishikawa, A. & Hirose, M. (2002) Arch. Toxicol. 76, 553-559. [DOI] [PubMed] [Google Scholar]
- 12.Busby, M. G., Jeffcoat, A. R., Bloedon, L. T., Koch, M. A., Black, T., Dix, K. J., Heizer, W. D., Thomas, B. F., Hill, J. M., Crowell, J. A. & Zeisel, S. H. (2002) Am. J. Clin. Nutr. 75, 126-136. [DOI] [PubMed] [Google Scholar]
- 13.Bloedon, L. T., Jeffcoat, A. R., Lopaczynski, W., Schell, M. J., Black, T. M., Dix, K. J., Thomas, B. F., Albright, C., Busby, M. G., Crowell, J. A. & Zeisel, S. H. (2002) Am. J. Clin. Nutr. 76, 1126-1137. [DOI] [PubMed] [Google Scholar]
- 14.Blake, C. C., Geisow, M. J., Oatley, S. J., Rerat, B. & Rerat, C. (1978) J. Mol. Biol. 121, 339-356. [DOI] [PubMed] [Google Scholar]
- 15.Blake, C. C., Swan, I. D., Rerat, C., Berthou, J., Laurent, A. & Rerat, B. (1971) J. Mol. Biol. 61, 217-224. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson, S. F., Rask, L. & Peterson, P. A. (1975) J. Biol. Chem. 250, 8554-8563. [PubMed] [Google Scholar]
- 17.Monaco, H. L., Rizzi, M. & Coda, A. (1995) Science 268, 1039-1041. [DOI] [PubMed] [Google Scholar]
- 18.Colon, W. & Kelly, J. W. (1992) Biochemistry 31, 8654-8660. [DOI] [PubMed] [Google Scholar]
- 19.Kelly, J. W., Colon, W., Lai, Z., Lashuel, H. A., McCulloch, J., McCutchen, S. L., Miroy, G. J. & Peterson, S. A. (1997) Adv. Protein Chem. 50, 161-181. [DOI] [PubMed] [Google Scholar]
- 20.Lai, Z., Colon, W. & Kelly, J. W. (1996) Biochemistry 35, 6470-6482. [DOI] [PubMed] [Google Scholar]
- 21.Lashuel, H. A., Lai, Z. & Kelly, J. W. (1998) Biochemistry 37, 17851-17864. [DOI] [PubMed] [Google Scholar]
- 22.Liu, K., Cho, H. S., Hoyt, D. W., Nguyen, T. N., Olds, P., Kelly, J. W. & Wemmer, D. E. (2000) J. Mol. Biol. 303, 555-565. [DOI] [PubMed] [Google Scholar]
- 23.Lashuel, H. A., Wurth, C., Woo, L. & Kelly, J. W. (1999) Biochemistry 38, 13560-13573. [DOI] [PubMed] [Google Scholar]
- 24.Quintas, A., Saraiva, M. J. & Brito, R. M. (1999) J. Biol. Chem. 274, 32943-32949. [DOI] [PubMed] [Google Scholar]
- 25.Quintas, A., Saraiva, M. J. & Brito, R. M. (1997) FEBS Lett. 418, 297-300. [DOI] [PubMed] [Google Scholar]
- 26.Westermark, P., Sletten, K., Johansson, B. & Cornwell, G. G., III (1990) Proc. Natl. Acad. Sci. USA 87, 2843-2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCarthy, R. E., III, & Kasper, E. K. (1998) Clin. Cardiol. 21, 547-552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saraiva, M. J., Costa, P. P. & Goodman, D. S. (1985) J. Clin. Invest. 76, 2171-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Plante-Bordeneuve, V. & Said, G. (2000) Curr. Opin. Neurol. 13, 569-573. [DOI] [PubMed] [Google Scholar]
- 30.Jacobson, D. R., Pastore, R. D., Yaghoubian, R., Kane, I., Gallo, G., Buck, F. S. & Buxbaum, J. N. (1997) N. Engl. J. Med. 336, 466-473. [DOI] [PubMed] [Google Scholar]
- 31.Holmgren, G., Ericzon, B. G., Groth, C. G., Steen, L., Suhr, O., Andersen, O., Wallin, B. G., Seymour, A., Richardson, S., Hawkins, P. N., et al. (1993) Lancet 341, 1113-1116. [DOI] [PubMed] [Google Scholar]
- 32.Suhr, O. B., Ericzon, B. G. & Friman, S. (2002) Liver Transpl. 8, 787-794. [DOI] [PubMed] [Google Scholar]
- 33.Dubrey, S. W., Davidoff, R., Skinner, M., Bergethon, P., Lewis, D. & Falk, R. H. (1997) Transplantation 64, 74-80. [DOI] [PubMed] [Google Scholar]
- 34.Yazaki, M., Tokuda, T., Nakamura, A., Higashikata, T., Koyama, J., Higuchi, K., Harihara, Y., Baba, S., Kametani, F. & Ikeda, S. (2000) Biochem. Biophys. Res. Commun. 274, 702-706. [DOI] [PubMed] [Google Scholar]
- 35.Sacchettini, J. C. & Kelly, J. W. (2002) Nat. Rev. Drug Discov. 1, 267-275. [DOI] [PubMed] [Google Scholar]
- 36.Bartalena, L. & Robbins, J. (1993) Clin. Lab. Med. 13, 583-598. [PubMed] [Google Scholar]
- 37.Adamski-Werner, S. L., Palaninathan, S. K., Sacchettini, J. C. & Kelly, J. W. (2004) J. Med. Chem. 47, 355-374. [DOI] [PubMed] [Google Scholar]
- 38.Baures, P. W., Oza, V. B., Peterson, S. A. & Kelly, J. W. (1999) Bioorg. Med. Chem. 7, 1339-1347. [DOI] [PubMed] [Google Scholar]
- 39.Baures, P. W., Peterson, S. A. & Kelly, J. W. (1998) Bioorg. Med. Chem. 6, 1389-1401. [DOI] [PubMed] [Google Scholar]
- 40.Johnson, S. M., Petrassi, H. M., Palaninathan, S. K., Mohamedmohaideen, N. N., Purkey, H. E., Nichols, C., Chiang, K. P., Walkup, T., Sacchettini, J. C., Sharpless, K. B. & Kelly, J. W. (2005) J. Med. Chem. 48, 1576-1587. [DOI] [PubMed] [Google Scholar]
- 41.Klabunde, T., Petrassi, H. M., Oza, V. B., Raman, P., Kelly, J. W. & Sacchettini, J. C. (2000) Nat. Struct. Biol. 7, 312-321. [DOI] [PubMed] [Google Scholar]
- 42.Oza, V. B., Smith, C., Raman, P., Koepf, E. K., Lashuel, H. A., Petrassi, H. M., Chiang, K. P., Powers, E. T., Sachettinni, J. & Kelly, J. W. (2002) J. Med. Chem. 45, 321-332. [DOI] [PubMed] [Google Scholar]
- 43.Peterson, S. A., Klabunde, T., Lashuel, H. A., Purkey, H., Sacchettini, J. C. & Kelly, J. W. (1998) Proc. Natl. Acad. Sci. USA 95, 12956-12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrassi, H. M., Klabunde, T., Sacchettini, J. & Kelly, J. W. (2000) J. Am. Chem. Soc. 122, 2178-2192. [Google Scholar]
- 45.Razavi, H., Powers, E. T., Purkey, H. E., Adamski-Werner, S. L., Chiang, K. P., Dendle, M. T. A. & Kelly, J. W. (2005) Bioorg. Med. Chem. 15, 1075-1078. [DOI] [PubMed] [Google Scholar]
- 46.Razavi, H., Palaninathan, S. K., Powers, E. T., Wiseman, R. L., Purkey, H. E., Mohamedmohaideen, N. N., Deechongkit, S., Chiang, K. P., Dendle, M. T., Sacchettini, J. C., et al. (2003) Angew. Chem. Int. Ed. Engl. 42, 2758-2761. [DOI] [PubMed] [Google Scholar]
- 47.Wiseman, R. L., Johnson, S. M., Kelker, M. S., Foss, T., Wilson, I. A. & Kelly, J. W. (2005) J. Am. Chem. Soc. 127, 5540-5551. [DOI] [PubMed] [Google Scholar]
- 48.Hammarström, P., Wiseman, R. L., Powers, E. T. & Kelly, J. W. (2003) Science 299, 713-716. [DOI] [PubMed] [Google Scholar]
- 49.Hammarström, P., Schneider, F. & Kelly, J. W. (2001) Science 293, 2459-2462. [DOI] [PubMed] [Google Scholar]
- 50.Ravindranath, M. H., Muthugounder, S., Presser, N. & Viswanathan, S. (2004) Adv. Exp. Med. Biol. 546, 121-165. [DOI] [PubMed] [Google Scholar]
- 51.Foss, T. R., Kelker, M. S., Wiseman, R. L., Wilson, I. A. & Kelly, J. W. (2005) J. Mol. Biol. 347, 841-854. [DOI] [PubMed] [Google Scholar]
- 52.Wiseman, R. L., Green, N. S. & Kelly, J. W. (2005) Biochemistry 44, 9265-9274. [DOI] [PubMed] [Google Scholar]
- 53.Purkey, H. E., Dorrell, M. I. & Kelly, J. W. (2001) Proc. Natl. Acad. Sci. USA 98, 5566-5571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hurshman, A. R., White, J. T., Powers, E. T. & Kelly, J. W. (2004) Biochemistry 43, 7365-7381. [DOI] [PubMed] [Google Scholar]
- 55.Schneider, F., Hammarström, P. & Kelly, J. W. (2001) Protein Sci. 10, 1606-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller, S. R., Sekijima, Y. & Kelly, J. W. (2004) Lab. Invest. 84, 545-552. [DOI] [PubMed] [Google Scholar]
- 57.Setchell, K. D., Brown, N. M., Desai, P., Zimmer-Nechemias, L., Wolfe, B. E., Brashear, W. T., Kirschner, A. S., Cassidy, A. & Heubi, J. E. (2001) J. Nutr. 131, 1362S-1375S. [DOI] [PubMed] [Google Scholar]
- 58.Liu, Y. & Hu, M. (2002) Drug Metab. Dispos. 30, 370-377. [DOI] [PubMed] [Google Scholar]