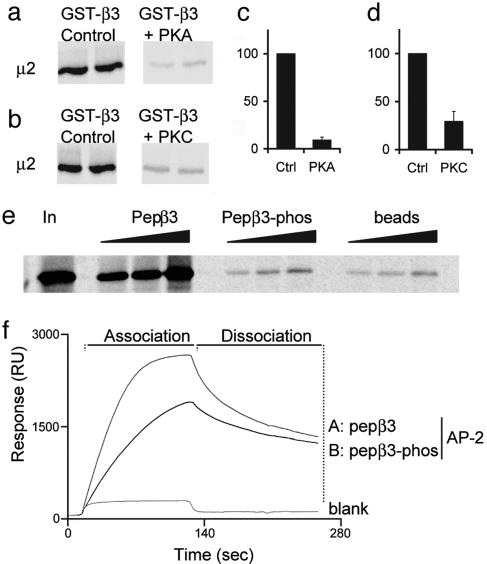
Fig. 3.
Binding of GABAAR β3 subunits to μ2 and AP2 is regulated by phosphorylation at serine residues S408 and S409. (a-d) GST-β3 was prephosphorylated in vitro by PKA (a and c) or PKC (b and d) and binding to 35S-labeled μ2 compared with nonphosphorylated GST-β3. (e) A peptide representing the μ2 binding domain in GABAAR β3 ICD pepβ3 (residues 401-412), binds with high affinity to 35S-labeled μ2, whereas an identical peptide, phosphorylated at serines S408 and S409 in this peptide, does not. Increasing amounts of peptide, pepβ3, and pepβ3-phos (coupled to beads via an N-terminal cysteine), or beads alone, were exposed to 35S μ2. Bound material was separated by SDS/PAGE and visualized by autoradiography. (f) Surface plasmon resonance analysis of the binding of pepβ3 and pepβ3-phos to native AP2 from brain.