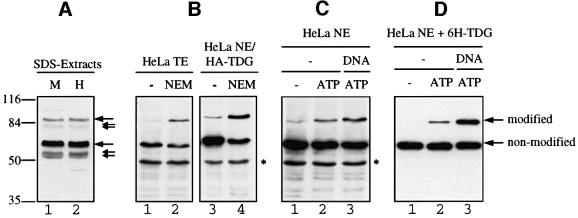
Fig. 2. TDG modification in cell extracts. Extracts of MRC5 and HeLa cells were subjected to SDS–PAGE and western blot analysis with specific polyclonal (A) and monoclonal (B, C and D) antibodies against human TDG. (A) Analysis of 10 µl of MRC5 (M) and HeLa (H) extracts prepared by direct lysis of 107 cells in SDS buffer. Immunostaining revealed two major TDG-specific signals at ∼60 and ∼86 kDa (large arrows) and a few minor signals representing faster migrating forms of the protein (small arrows). (B) Total cell extract (TE) from HeLa cells or nuclear extract (NE) from HeLa cells overexpressing HA-tagged TDG were prepared in the absence or presence of 5 mM NEM. A 100 µg aliquot of TE and 50 µg of NE proteins were analysed by western blotting with antibodies against TDG (lanes 1 and 2) and against the HA tag (lanes 3 and 4). In both cases, the 86 kDa but not the 60 kDa form of TDG appeared stronger when the cell extracts were prepared in the presence of NEM. The asterisk indicates an unspecific protein detected by the secondary anti-rat IgG antibody used. (C) HeLa nuclear extract (NE) prepared in the absence of NEM showed only a faint band of the 86 kDa form of TDG (lane 1). De novo modification of endogenous TDG was detectable following a short incubation of the extract with 10 mM ATP (lane 2). This reaction was stimulated further by the presence of a 60mer G·U heteroduplex DNA substrate (lane 3). (D) Recombinant His6-tagged TDG (6H-TDG) was incubated with HeLa nuclear extract in the absence or presence of 10 mM ATP and G·U mismatched DNA. Western blotting revealed that the recombinant protein was modified in vitro in an ATP-dependent manner (lanes 1 and 2), and that this reaction was stimulated significantly when TDG was pre-bound to a 60mer G·U heteroduplex substrate (lane 3).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.