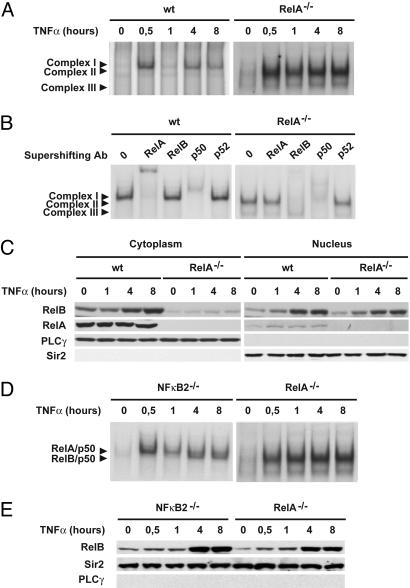
Fig. 1.
Absence of RelA leads to rapid and sustained RelB activation in TNF-α-stimulated MEFs. (A) Nuclear extracts from WT and RelA-deficient MEFs treated with TNF-α for the indicated periods of time were analyzed by EMSA using a 32P-labeled HIV-LTR tandem κB oligonucleotide as a probe. (B) For supershift, nuclear extracts from WT and RelA-deficient MEFs treated with TNF-α for 8 h were incubated with the indicated antibodies before incubation with the labeled probe. Complex I, RelA/p50; complex II, RelB/p50; complex III, p50/p50. (C) Cytoplasmic and nuclear extracts of WT and RelA-deficient MEFs treated with TNF-α for the indicated periods of time were analyzed by immunoblotting for the indicated proteins. Phospholipase C-γ-1 (PLCγ) and Sir2 were used as quality controls to verify the absence of cytoplasmic contamination in nuclear extracts and nuclear contamination in cytoplasmic extracts, respectively. (D) Nuclear extracts from RelA- and NF-κB2-deficient MEFs treated with TNF-α for the indicated periods of time were analyzed by EMSA as described in A.(E) Nuclear extracts from RelA- and NF-κB2-deficient MEFs treated with TNF-α for the indicated periods of time were analyzed by immunoblotting for the indicated proteins.