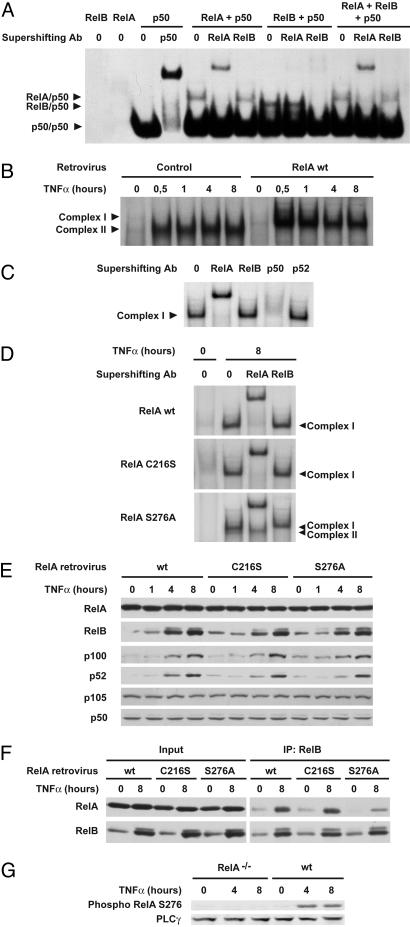
Fig. 3.
RelA serine-276 is critical for RelA/RelB complex formation and consequent inhibition of RelB DNA binding. (A) RelA inhibits RelB/p50 DNA binding in vitro. The κB probe was incubated with the indicated in vitro translated NF-κB proteins, and DNA binding was analyzed by EMSA. For supershift, in vitro translated NF-κB proteins were incubated with the indicated antibodies before incubation with the labeled probe. (B) Reexpression of RelA in RelA-deficient MEFs abolishes TNF-α-induced RelB/p50 DNA binding. Nuclear extracts from RelA-deficient MEFs stably transduced with retroviruses encoding either the parental empty vector (control) or WT RelA and treated with TNF-α for the indicated periods of time were analyzed by EMSA. (C) For supershift, nuclear extracts from RelA-deficient MEFs reexpressing WT RelA treated with TNF-α for 8 h were incubated with the indicated antibodies before incubation with the labeled probe. (D) RelA serine-276 is critical for inhibition of TNF-α-induced RelB DNA binding. Nuclear extracts from RelA-deficient MEFs stably transduced with the indicated RelA retroviruses, either untreated or treated with TNF-α for 8 h, were analyzed for NF-κB activity by EMSA. For supershift, nuclear extracts were incubated with the indicated antibodies before incubation with the labeled probe. (E) Protein expression levels of NF-κB family members in RelA-deficient MEFs reexpressing WT RelA or C216S or S276A RelA mutants. Whole-cell extracts from RelA-deficient MEFs infected with the indicated RelA retroviruses and treated with TNF-α for the indicated periods of time were analyzed by immunoblotting for the indicated proteins. (F) RelA serine-276 is critical for association with RelB. Whole-cell extracts from RelA-deficient MEFs infected with the indicated RelA retroviruses, either untreated or treated with TNF-α for 8 h, were subjected to immunoprecipitation (IP) with anti-RelB antibody and analyzed for associated RelA. (G) TNF-α induces RelA serine-276 phosphorylation. Whole-cell extracts from either WT MEFs or RelA-deficient MEFs treated with TNF-α for the indicated periods of time were analyzed by immunoblotting for RelA serine-276 phosphorylation.