Abstract
Estrogenic effects have been implicated in sexual differentiation of brain and behavior, in part by affecting neuronal activity in the ventromedial nucleus of the hypothalamus (VMN). We report here a remarkable sex difference in estrogenic regulation of neuronal activity in male vs. female neural networks. Spontaneous synaptic currents originating from a population of neurons were recorded in primary VMN cultures using the whole-cell patch-clamp technique. Treatment with 17β-estradiol (E2, 10 nM) for 24 h induced opposite effects in the two sexes: the frequency of spontaneous synaptic events decreased significantly in neurons derived from males but increased in those from females. Interestingly, the 24-hour E2 effect was partially reversed by an acute application (5 min) of a second dose of E2 (10 nM), suggesting an interaction between extended (24-hr) and acute (5-min) effects of E2 in VMN neurons. To understand the underlying mechanism of this sexually dimorphic action of E2, we analyzed the E2 effect on GABAergic neurotransmission by recording miniature inhibitory postsynaptic currents. After 24-hour E2 treatment, both the amplitude and frequency of miniature inhibitory postsynaptic currents increased in neurons derived from males but decreased in those from females. These results suggest that E2-induced changes in GABAergic inhibition could at least partially explain E2 effects on neuronal activity. We conclude that E2 may have sexually dimorphic effects on the synaptic output of VMN neurons by modulating GABAergic neurotransmission.
Keywords: GABA, sexual dimorphism, steroid hormone
Estrogens (E) play important roles in a variety of neurobiological processes, including neural development and adult behaviors (1). The functional outcomes of these processes are coordinated with physiological events that are fundamentally different in males (♂) and females (♀). These physiological events involve E interactions with nuclear E receptors (ERs), as well as interactions at several cellular levels, including signal transduction systems. One focus of these interactions is on hypothalamic neuronal activity. During a perinatal sensitive period, E exposure enhances neuronal excitability in the ♂ hypothalamus by affecting amino acid neurotransmitters, which might be a crucial mechanism in the process of masculinization of the ♂ brain (2). E plays a fundamental role in lordosis via actions in the ventromedial nucleus of the hypothalamus (VMN) (3, 4). Previous studies (5, 6) have demonstrated that an increase in VMN neuronal activity is a mechanism by which E acts through VMN to induce lordosis. However, the questions of exactly how E increases VMN neuronal activity and how sex differences might arise here remain to be elucidated.
GABA plays an important role in VMN development (7), and GABAergic neurotransmission has been implicated in E's effects on several important functions, such as sexual differentiation during neural development (8) and lordosis behavior (9). E has been shown to affect many aspects of GABAergic transmission, including GABA turnover rate (10), GABA synthesis enzyme glutamic acid decarboxylase (GAD) mRNA, and protein levels in the hypothalamus (11). E also regulates GABA receptor expression (2) and subunit composition (12). Therefore, a possible mechanism for mediating E's effects in VMN could be through modulating GABAergic neurotransmission. To investigate how estradiol regulates neuronal activity, primary neuronal cultures of VMN from both ♂ and ♀ rats were used here to examine the effect of E treatment on neuronal activity and GABAergic neurotransmission.
Methods
Cell Culture. VMN neuronal cultures were prepared with procedures similar to a previous report, except the dissected brain area (13). A more detailed description of dissecting VMN neurons and cultural procedures can be found in Supporting Text, which is published as supporting information on the PNAS web site. Briefly, brains were taken from postnatal day 1 Sprague-Dawley rat pups. The VMN of both sides were dissected out and incubated for 30 min in 0.05% trypsin/EDTA solution (pH 7.2). After enzyme treatment, tissue blocks were triturated gently into dissociated cells and plated onto a monolayer of astrocytes. The culture medium (500 ml) contained MEM without phenol red (GIBCO), 5% FBS, 10 ml of B-27 supplement, 100 mg of NaHCO3, 20 mM d-glucose, 0.5 mM l-glutamine, and 25 units/ml penicillin/streptomycin. Cells were maintained in an incubator at 37°C and 5% CO2 for up to 2-3 weeks.
E Treatment. VMN neurons were given either 24-hr or 5-min 17β-estradiol (E2, water soluble, 10 nM, Sigma) treatment. For 24-hr treatment, E2 diluted in culture medium was added in the cultures 24 h before recording, and the same amount of culture medium was added in the control group. For acute treatment, E2 diluted in a bath solution was perfused for 5 min after recording baseline activity. To maintain strict internal controls, ♂ and ♀ pups were collected at the same time, prepared at the same cell density, and treated with E2 on the same day for the same duration. Every experiment was repeated in at least three different batches of cultures. Sister coverslips in the same batch of culture were used either as control or E2 treatment. In addition, all of the recording conditions were strictly the same for ♂ and ♀ neurons. The same duration of data acquisition (4 min for spontaneous activity, 2 min for miniature postsynaptic currents) within each different condition was chosen for data analysis to avoid any sampling error.
Electrophysiology. Patch-clamp recordings of primary cultured VMN neurons were made between 12 and 16 days in vitro, when GABAergic action is largely inhibitory (14). Whole-cell recordings were performed by using MultiClamp 700A amplifier (Axon Instruments, Foster City, CA). The pipette tip resistance was 2-4 MΩ. The recording chamber was continuously perfused with a bath solution of 128 mM NaCl/30 mM d-glucose/25 mM Hepes/5 mM KCl/2 mM CaCl2/1 mM MgCl2 (pH 7.3, adjusted with NaOH). Tetrodotoxin (TTX, 0.5 μM) and cyano-2, 3-dihydroxy-7-nitroquinoxaline (CNQX, 10 μM) were added into the bath solution when miniature inhibitory postsynaptic current (mIPSCs) were recorded. The pipette solution contained 147 mM KCl, 5 mM disodium phosphocreatine, 2 mM EGTA, 10 mM Hepes, 4 mM MgATP, 0.6 mM Na2GTP, and 20 units/ml phosphocreatine kinase (pH 7.3, adjusted with KOH). GABA, TTX, CNQX, MgATP, Na2GTP, disodium phosphocreatine, and phosphocreatine kinase were purchased from Sigma). All drugs were diluted in a fresh bath solution to final concentration before experiments. The series resistance was typically 10-20 MΩ; data were not included if changes were >30%. The holding potential was set at -70 mV. Data were acquired by pclamp 9.0 software and analyzed by clampfit software (Axon Instruments). Spontaneous and mIPSC events were analyzed by using minianalysis software (Synaptosoft, Decatur, GA).
Statistics. Population events were collected from a group of neurons to assess total synaptic output of a neural network. To ensure that the information from every event is counted, the Kolmogorov-Smirnov test was used for statistical analysis to compare overall synaptic activity changes (15). The distributions of all events within each recorded cell were analyzed to make sure they would fit a Poisson distribution. Frequency and amplitude of each group were expressed by median values of all events within the exact same length of recording (see above) in each group. For GABA-evoked whole-cell currents, which originated only from the recorded neuron, Student's t test was used for statistical comparison.
Results
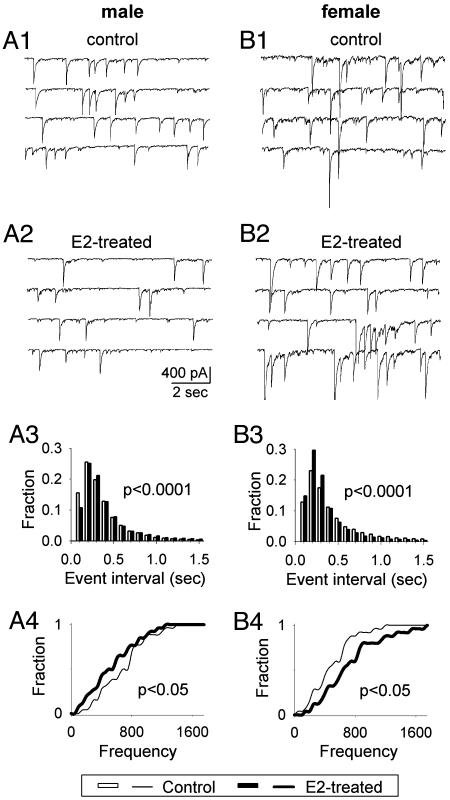
E2 Treatment Induced Opposite Effects on Spontaneous Synaptic Activity in VMN Neurons Derived from ♂ vs. ♀. We examined the effect of E2 on the total spontaneous synaptic events that originated from VMN neurons in culture. We first tested the effect of 24-hr treatment of E2 (10 nM) in cultured VMN neurons. Interestingly, the 24-hr E2 treatment resulted in opposite effects on neuronal activity in ♂ vs. ♀ VMN neurons. Fig. 1 shows typical examples of spontaneous synaptic activity recorded from ♂ and ♀ VMN neurons respectively under whole-cell voltage-clamp condition (holding potential = -70 mV). In ♂ VMN neurons, the frequency of spontaneous synaptic activity significantly decreased (Fig. 1 A1 and A2), but in ♀ neurons, the frequency significantly increased (Fig. 1 B1 and B2). Pooled data in histograms summarized the overall changes of total spontaneous synaptic activity in ♂ (Fig. 1 A3) and ♀ neurons (Fig. 1B3). For ♂ neurons, the interevent interval increased after 24-hr E2 treatment, as reflected by a decrease of small event interval and an increase of large event interval (Fig. 1 A3). After converting the interevent interval (sec) into frequency (Hz), the median value of the frequency of spontaneous synaptic activity in the E2-treated group was 2.92 Hz, a 31% decrease compared to the control group (4.18 Hz; P < 0.0001). In ♀ VMN neurons (Fig. 1B3), however, the frequency of spontaneous synaptic activity increased by 25% in the 24-hr E2-treated group (4.63 Hz) compared with the control group (3.70 Hz; P < 0.0001). The frequency increase was reflected by a left shift of the interevent interval at the histogram (Fig. 1B3). In addition to the population synaptic events, the Kolmogorov-Smirnov test was also performed based on the average value of each cell to further evaluate the significance of E2's effect at the individual cell level. For ♂ neurons (n = 26), the frequency of spontaneous synaptic activity significantly decreased after 24-hr E2 treatment (Fig. 1 A4, P < 0.05). For ♀ neurons (n = 25), the frequency significantly increased in E2-treated group (Fig. 1B4, P < 0.05). This sex difference in the change of spontaneous synaptic activity after 24-hr E2 treatment reveals that E2 has a sexually dimorphic effect in developing neurons.
Fig. 1.
E2 treatment (24 h) differentially alters spontaneous synaptic activity in ♂ and ♀ VMN neurons. (A1 and A2) Recording traces show E2 treatment (10 nM) decreased the frequency of spontaneous synaptic activity in a ♂ neuron. (B1 and B2) Recording traces show the same E2 treatment increased the frequency of spontaneous synaptic activity in a ♀ neuron. (A3 and B3) Histograms showing the pooled data of overall changes within each group in spontaneous synaptic activity before and after E2 treatment. A3 shows that events with small interevent interval decreased in ♂ neurons after E2 treatment, indicating a decrease in the frequency of spontaneous synaptic activity (P < 0.0001, Kolmogorov-Smirnov Test). B3 shows a significant increase in the frequency of spontaneous synaptic activity (increase of events with small intervals) in ♀ neurons (P < 0.0001). (A4 and B4) Cumulative histograms show group data based on the average frequency of each individual cell. The frequency of spontaneous synaptic activity was significantly decreased in ♂ (P < 0.05) but increased (P < 0.05) in ♀ VMN neurons after E2 treatment.
The 24-hr E2 treatment also changed the amplitude of spontaneous synaptic currents (Fig. 2). In ♂ neurons, the median amplitude of all spontaneous events increased from 55.94 pA in the control group to 59.20 pA in the E2-treated group (Fig. 2 A1; P < 0.0001). In ♀ neurons, the change is larger with the median amplitude at 56.76 pA for the control group and 65.92 pA for the E2-treated group (Fig. 2B1; P < 0.0001). Moreover, when taking a closer look at the large (largest 10%) and small (lowest 10%) events, it appears that E2's effect is more apparent in increasing the amplitude of large events. Fig. 2 A2 and B2 show that the amplitude of the largest 10% events increased dramatically in both ♂ (control 264 pA, E2-treated 526 pA, P < 0.0001) and ♀ (control 358 pA; E2-treated 828 pA; P < 0.0001) neurons. In contrast, the amplitude of the lowest 10% events did not show much difference between the control and E2-treated group in both ♂ (control, 31.74 pA; E2-treated, 32.35 pA) and ♀ neurons (control, 31.72 pA; E2-treated, 32.43) (Fig. 2 A3 and B3). Those large events are most likely evoked by action potentials, suggesting a significant effect of E2 on neuronal firing rate.
Fig. 2.
E2 treatment (24 h) increases the amplitude of spontaneous synaptic activity in both ♂ and ♀ VMN neurons. (A and B) Histograms illustrate the overall spontaneous synaptic activity amplitude before and after E2 treatment (10 nM) in ♂ and ♀ neurons: all events (A1 and B1), largest 10% events (A2 and B2), and lowest 10% events (A3 and B3). (A1 and B1) Of all events, spontaneous synaptic activity amplitude was increased in both ♂ (median of control, 55.94 pA; E2 treated, 59.20 pA; P < 0.0001) and ♀ neurons (median of control, 56.76 pA; E2 treated, 65.92 pA; P < 0.0001). Of the largest 10% events, the amplitude showed more significant increase by E2 treatment in ♂ (A2, control, 264 pA; E2 treated, 526 pA; P < 0.0001) and ♀ neurons (B2, control, 358 pA; E2 treated, 828 pA; P < 0.0001). Of the lowest 10% events, the amplitude showed less significant difference in ♂ (A3, control, 31.74 pA; E2 treated, 32.35 pA; P < 0.05) and ♀ neurons (B3, control, 31.72 pA; E2 treated, 32.43 pA; P < 0.0001).
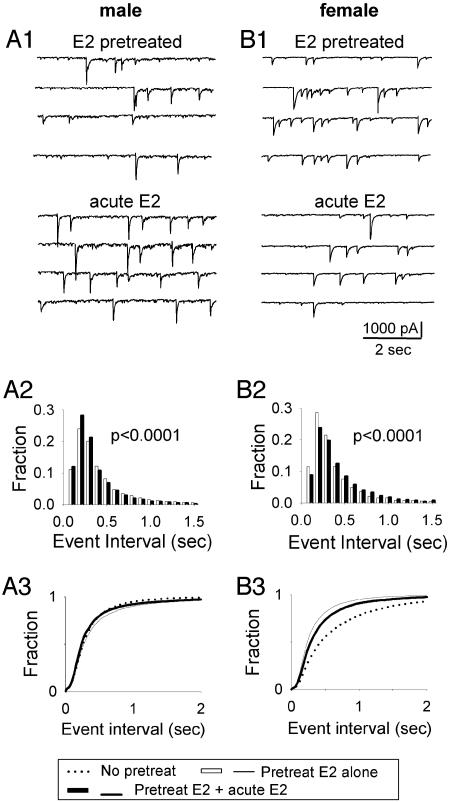
The 24-Hour E2 Effects Were Partially Reversed by Acute Application of a Second Dose of E2 in VMN Neurons Derived from both ♂ and ♀. We tested whether E2 has any acute effect on neuronal activity in cultured VMN neurons and found no significant change in spontaneous synaptic activity after acute E2 treatment (10 nM, 5 min) in both ♂ and ♀ VMN neurons (data not shown). However, when 10 nM E2 was applied acutely (5 min) after 24-hr pretreatment with E2 (10 nM), a significant change in spontaneous synaptic activity was revealed. Fig. 3 A1 and B1 show typical acute E2 effects after 24-hr E2 pretreatment, which are exactly opposite to the 24-hr E2 effects in both ♂ and ♀ neurons. Quantitative analysis shows that when ♂ neurons were challenged with acute E2 for 5 min after 24-hr E2 pretreatment, the frequency of spontaneous currents (4.26 Hz) increased by 22% compared with 24-hr E2 pretreatment alone (3.47 Hz) (Fig. 3A2, P < 0.0001). The cumulative histogram in Fig. 3A3 shows that 24-hr E2 treatment alone (thin solid line) decreased the frequency of spontaneous synaptic activity (right shift) compared with the control group (no E2 treatment, dotted line). In contrast, the acute application of a second dose of E2 (thick solid line) reversed this 24-hr E2 effect by increasing the frequency (left shift). In ♀ neurons, both the 24-hr E2 treatment and the acute E2 effect after 24-hr treatment showed opposite changes as those in ♂ neurons. As shown in Fig. 3B2, the frequency of spontaneous synaptic activity in ♀ neurons decreased by 18% with the acute E2 application after 24-hr E2 pretreatment (3.51 Hz) compared with 24-hr E2 pretreatment alone (4.26 Hz, P < 0.0001). This decrease of activity by acute E2 (thick line in Fig. 3B3) partially reversed the 24-hr E2 effect (thin line), which increased the frequency of spontaneous synaptic activity in ♀ neurons, as shown by the cumulative plot in Fig. 3B3.
Fig. 3.
After 24-hr E2 pretreatment, a second dose of acute (5 min) E2 (10 nM) treatment altered both frequency and amplitude of spontaneous activity. (A) Recording traces (A1) and histograms (A2) show that acute E2 treatment significantly increased the frequency (A2, P < 0.0001) of spontaneous synaptic activity after 24-hr E2 pretreatment in ♂ neurons. (B) Recording traces (B1) and histograms (B2) show acute E2 treatment significantly decreasing the frequency (B2, P < 0.0001) after 24-hr E2 pretreatment in ♀ neurons. (A3 and B3) Cumulative plot shows that acute E2 treatment (black thick line) at least partially reversed 24-hr E2 treatment effects (thin line) on the frequency of spontaneous synaptic activity in both ♂ and ♀ neurons.
The acute E2 treatment also changed the amplitude of the spontaneous synaptic activity. That is, when a second dose of E2 was applied after 24-hr E2 (10 nM) pretreatment, the amplitude of spontaneous synaptic activity was slightly increased in ♂ neurons (E2 pretreatment alone, 53.10 pA; acute E2 after pretreatment, 56.15 pA; P < 0.0001) but significantly decreased in ♀ neurons (E2 pretreatment alone, 62.26 pA; acute E2 after pretreatment, 53.71 pA; P < 0.0001).
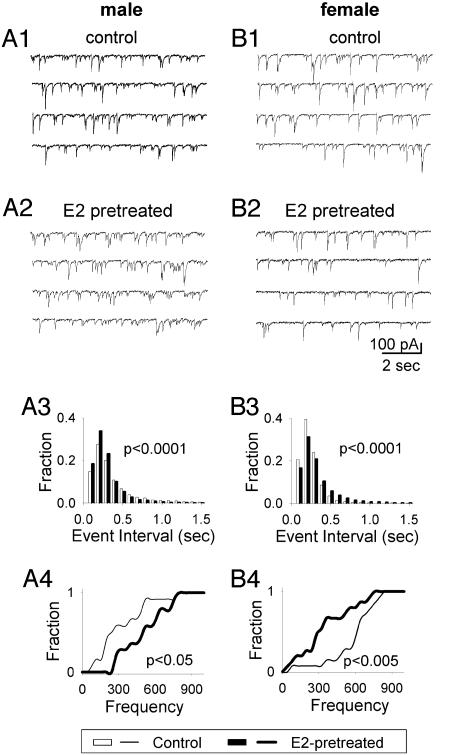
Twenty-Four-Hour and Acute E2 Treatment Differentially Changed the mIPSCs in VMN Neurons Derived from ♂ and ♀. To investigate underlying mechanisms of the sexual dimorphic E2 effects on spontaneous synaptic activity, we recorded mIPSCs in the presence of tetrodotoxin (0.5 μM) and cyano-2, 3-dihydroxy-7-nitroquinoxaline (10 μM) to examine E2 effects on GABAergic neurotransmission. mIPSCs were abolished by GABAA receptor antagonist bicuculline (20 μM, data not shown). Fig. 4 A1-A2 and B1-B2 show typical recordings of mIPSCs in ♂ and ♀ neurons with and without 24-hr E2 treatment. Interestingly, the frequency of mIPSCs increased in ♂ neurons, but decreased in ♀ neurons after 24-hr E2 treatment. For all ♂ neurons recorded (n = 27) (Fig. 4A3), the interevent interval was significantly reduced after E2 pretreatment, indicating that the frequency of mIPSCs was significantly increased (control, 4.48 Hz; E2-treated group, 5.21 Hz; P < 0.0001). In contrast, for all ♀ neurons recorded (n = 29) (Fig. 4B3), the interevent interval increased, and therefore the frequency of mIPSCs was significantly decreased after E2 pretreatment (control, 5.75 Hz; E2-treated group, 4.78 Hz; P < 0.0001). To further examine E2's effect on mIPSCs at the individual cell level, the Kolmogorov-Smirnov test was performed based on the average frequency of each individual cell. The frequency of mIPSCs still showed a significant increase in ♂ neurons (Fig. 4A4, n = 27, P < 0.05) but a decrease in ♀ neurons (Fig. 4B4, n = 29, P < 0.005). Because our VMN neurons are cultured for ≈2 weeks from neonatal pups, GABA exerts inhibitory function at this stage (14). Thus, an increase of mIPSC frequency implies an increase of presynaptic GABA release, which will inhibit neuronal activity. This explains, at least partially, that in ♂ neurons, 24-hr E2 treatment increased the mIPSC frequency but decreased that of spontaneous synaptic activity, whereas in ♀ neurons, 24-hr E2 treatment decreased the mIPSC frequency but increased the spontaneous synaptic activity. These results suggest that the E2 effect on the overall neural network activity could be caused at least partly by the E2 effect on GABAergic neurotransmission.
Fig. 4.
E2 treatment (24 h) differentially changed mIPSCs in ♂ vs. ♀ neurons. (A1 and A2) Representative traces show spontaneous synaptic activity in control (A1) and E2 treatment (A2) in ♂ neurons. (A3) Histogram of total synaptic event interval shows that 24-hr E2 treatment significantly decreased the event interval (P < 0.0001) in ♂ neurons. (A4) Cumulative plot based on the averaged value of individual neurons shows a significant increase of the mIPSC frequency (P < 0.05) in ♂ neurons. (B) Recording traces (B1 and B2), histogram (B3), and cumulative plot (B4) show that 24-hr E2 treatment significantly decreased the mIPSC frequency (B3, P < 0.0001; B4, P < 0.005) in ♀ neurons.
The 24-hr E2 treatment also affects the amplitude of mIPSCs. The mIPSC amplitude was increased in ♂ neurons by 10% (control, 25.64 pA; E2-treated, 28.08 pA; P < 0.0001) but decreased in ♀ neurons by 19% (control, 35.40 pA; E2-treated, 28.70 pA; P < 0.0001), compared with their control groups, respectively.
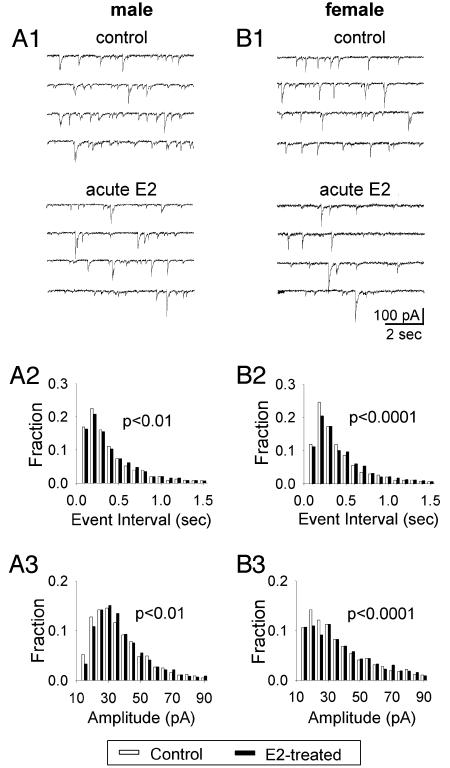
We also examined the acute E2 effect on mIPSCs. Fig. 5 A1 and B1 show examples of mIPSC recordings in ♂ and ♀ VMN neurons. Acute E2 application (10 nM, 5 min) slightly decreased the frequency of mIPSCs in both ♂ (Fig. 5A2) (control, 3.86 Hz; acute E2, 3.61 Hz; P < 0.01) and ♀ neurons (Fig. 5B2) (control, 3.64 Hz; acute E2, 3.30 Hz; P < 0.0001). Nevertheless, the amplitude of mIPSCs (Fig. 5 A3 and B3) showed slight increases in both ♂ (Fig. 5A3) (control, 31.13 pA; acute E2, 32.35 pA; P < 0.01) and ♀ neurons (Fig. 5B3) (control, 31.13 pA; acute E2, 34.79 pA; P < 0.0001) by acute E2 application. The opposite effect of acute E2 application on the amplitude and frequency of mIPSCs might explain why acute E2 did not show a significant effect on the overall spontaneous synaptic activity.
Fig. 5.
Acute (5-min) E2 treatment alone changed mIPSCs in ♂ and ♀ neurons. (A) Recording traces (A1) and histograms (A2 and A3) show that acute E2 treatment significantly increased the event interval and hence decreased the frequency (A2; P < 0.01) but increased the amplitude (A3; P < 0.01) in ♂ neurons. (B) Recording traces (B1) and histograms (B2 and B3) show that acute E2 treatment significantly decreased mIPSCs frequency (B2; P < 0.0001) but increased the amplitude (B3; P < 0.0001) in ♀ neurons.
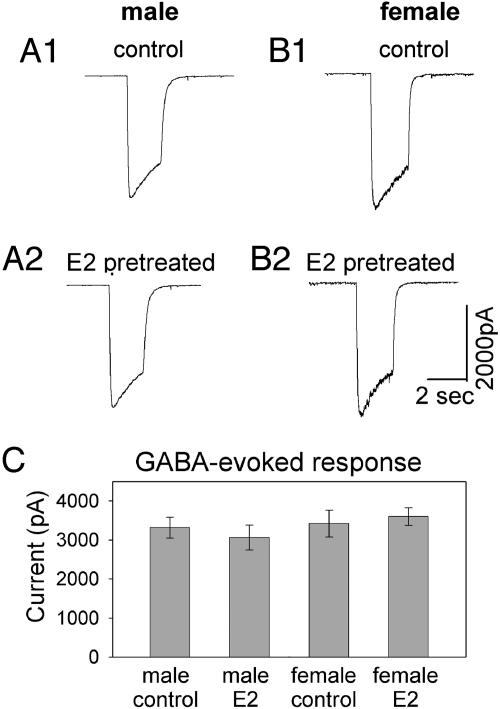
The 24-Hour E2 Treatment Did Not Affect the GABA-Evoked Whole-Cell Currents in VMN Neurons Derived from both ♂ and ♀. E2 effects on mIPSC amplitude suggest that synaptic GABAA receptors are modulated by E2. In addition to synaptically localized GABAA receptors, there are also many GABAA receptors located at extrasynaptic membranes. To examine whether all GABAA receptors on cell membranes are affected by E2 treatment, GABA-evoked whole-cell currents were recorded by a bath application of GABA (20 μM) to activate both synaptic and extrasynaptic receptors. Fig. 6 A1-A2 and B1-B2 show typical examples of GABA-evoked whole-cell currents in ♂ and ♀ VMN neurons with or without 24-hr E2 treatment correspondingly. Fig. 6C shows the average amplitudes of GABA-evoked currents in each group. There was no significant difference between the control and E2-treated groups in either ♂ (control, mean ± SE, 3320 ± 264; E2-treated, 3064 ± 320; n = 26; P > 0.2, Student's t test) or ♀ neurons (control, 3423 ± 345; E2-treated, 3603 ± 2250; n = 26; P > 0.4). This result indicates that E2 treatment does not affect all GABAA receptors on cell membranes.
Fig. 6.
Twenty-four-hour E2 treatment did not change GABA-evoked whole-cell currents. (A and B) Recording traces show the absence of E2 effect on GABA-evoked whole-cell currents in ♂ and ♀ neurons. (C) Quantitative analysis illustrating similar amplitudes of GABA-evoked membrane currents within each group (n = 26). There was no significant difference between the control and E2-treated groups in either ♂ (P > 0.2) or ♀ (P > 0.4) neurons.
Discussion
Sex Differences in E Regulation of VMN Neuronal Activity. VMN modulates several homeostatic, neuroendocrine, and behavioral functions. In ♀, VMN controls female-typical sexual behaviors (3, 4, 16). Morphologically, the hypothalamus participates in sexually dimorphic forebrain circuits (17). Morphological sex differences are also evident at the single-cell level (18). Our results show that in response to E2 treatment, there is a striking difference in synaptic activity changes between ♂ and ♀ VMN neurons. This sex difference of E2 regulation of neuronal activity in VMN neurons may contribute to functional sex differences in VMN.
Many E effects are mediated by classic ERs. ERs were shown to be present in the hypothalamus even before birth (19), making it possible for E to exert organizational effects on the brain during the perinatal sensitive period (8). VMN is one of the most concentrated brain areas for ER's expression (20, 21). Hybridization signals of ER mRNA in ♂ VMN were lower compared with those in both intact and ovariectomized ♀ rats (22). Moreover, E can influence ER mRNA expression in VMN in a sex-dependent way (23). We speculate that the sex difference of E regulation of neuronal activity may be related to sex differences in E-influenced ER expression.
Orchestration of Acute and Extended Effects of E. There is accumulating evidence of E effects via nonclassic actions (24, 25). These rapid effects of E indicate the possibility of nongenomic effects. A two-pulse E paradigm showed that a membrane E action potentiated a nuclear E action in a nerve cell line (26). This inspired us to test directly the possible interactions between the acute and extended effect of E2 in VMN neurons. We found that when cultured neurons were challenged with an acute E2 application after 24-hr E2 treatment, there was a rapid E2 effect on neuronal activity in a sexually dimorphic manner. Our results support a possibility of coordination between genomic and nongenomic E effects in the VMN, but mechanisms remain obscure.
Interactions Between E and GABAergic Neurotransmission in VMN. Increasing evidence suggests that E interacts with GABAergic neurotransmission in many brain areas. Gonadal hormones alter GABA synthesis and metabolites in the hypothalamus, including the GABA level (27), GAD messenger RNA level (11), and GABA receptors (24, 28). As early as embryonic day 13, cells and fibers containing GABA and GAD67 encircled the primordial VMH area. At the same time, cells containing ERα were also seen in the developing VMH (29). During development, a sex difference in GAD messenger RNA (30) and GABA neurotransmitter levels (31) was observed in the hypothalamic and limbic areas. E has the ability to alter mRNA levels of GAD65 and GAD67 and the proportion of cells expressing GAD (11). In our study, a clear effect of E2 treatment on mIPSC frequency was found in both ♂ and ♀ VMN neurons. This indicates a significant presynaptic regulation of E2 on GABAergic neurotransmission.
E also acts postsynaptically by modulating GABAA receptors and hence changing the amplitudes of mIPSCs. VMN neurons from neonatal rats revealed significant sex-specific differences in the kinetics of GABAA agonist-induced currents (28). In addition, E2 has been shown to enhance GABA-induced intracellular calcium before full neuronal maturation (32). In a recent study (33), E2 was found to rapidly modulate the function of GABAB receptors in hypothalamic neurons. Besides GABA receptors, second-messenger pathways might also be involved in the postsynaptic mechanisms in a sex dimorphic way. Activation of GABAA receptors differentially modulated CREB phosphorylation in the hypothalamus of newborn ♂ and ♀. The response to GABA can be excitatory or inhibitory on signaling pathways, depending on the sex (34).
Our data show an interesting pattern: that changes in the overall neuronal activity are negatively correlated to those of mIPSCs, consistent with the notion that GABA function switches into an inhibitory function in these hypothalamic neurons (14). Interactions between E2 and GABAergic neurotransmission system during the critical period are important mediators of sexual differentiation in the basal forebrain (2). Specifically, the mRNA level of GAD is twice as high in some steroid-concentrating regions of the hypothalamus in the neonatal ♂ brain compared with ♀ (30). Early in development, GABA is actually excitatory and induces depolarization in neurons (14), resulting in elevated intracellular calcium levels (35). The enhanced and prolonged excitatory GABAergic input should result in substantially higher levels of neuronal excitation in the ♂ brain (2). This increased level of neuronal excitation is proposed to be a potential mechanism mediating the permanent masculinization of the brain (2). In the current study, we found apparently higher GABA input (manifested by increased mIPSC frequency) but decreased neuronal activity in ♂ neurons. Because the primary cultures used in our study were kept at least 12 days in vitro, GABA action may have been transformed from excitation into inhibition at this age (14), which would be different from early development in vivo in the neonatal brain (8). Therefore, although stronger GABAergic input in immature neurons resulted in higher neuronal excitation in ♂ when GABA is excitatory (8), in our culture condition, the increased GABAergic input is inhibitory and hence decreases the neuronal activity in ♂ neurons.
Regarding roles of VMN GABAergic neurotransmission in lordosis behavior in the adult, both positive (36, 37) and negative (38, 39) effects can be observed. There may be interactions with other neurotransmission systems (40). Our results here found that 24-hr E2 treatment decreased mIPSCs and increased the neuronal activity in ♀ VMN cultures, which may suggest a negative link between GABAergic neurotransmission and neuronal activity or behavior. These data would be consistent with previous studies suggesting an estrogenic regulation of lordosis by changing the excitation/inhibition balance in VMN (41).
Supplementary Material
Acknowledgments
This work is supported by National Institutes of Health Grant HD-05751 (to D.W.P.), the Pennsylvania State University Life Science Consortium Innovative Biotechnology Research Fund grant, Pennsylvania State University Eberly College of Science Special Fund, and National Science Foundation Grant 0236429 (to G.C.).
Abbreviations: E, estrogen; E2, 17β-estradiol; GAD, glutamic acid decarboxylase; mIPSC, miniature inhibitory postsynaptic current; VMN, ventromedial nucleus of the hypothalamus; ER, estrogen receptor.
References
- 1.Pfaff, D. W., Arnold, A., Etgen, A. M., Fahrbach, S. E. & Rubin, R. T. (2002) Hormones, Brain and Behavior (Academic, San Diego).
- 2.McCarthy, M. M., Davis, A. M. & Mong, J. A. (1997) Brain Res. Bull. 44, 487-495. [DOI] [PubMed] [Google Scholar]
- 3.Pfaff, D. W. (1980) Estrogen and Brain Function, Neural Analysis of a Hormone Controlled Mammalian Reproductive Behavior (Springer, New York).
- 4.Pfaff, D. W. (1999) Drive: Neurobiological and Molecular Mechanisms of Sexual Motivation (MIT Press, Cambridge, MA).
- 5.Pfaff, D. W. & Sakuma, Y. (1979) J. Physiol. 288, 189-202. [PMC free article] [PubMed] [Google Scholar]
- 6.Harlan, R. E., Shivers, B. D., Kow, L. M. & Pfaff, D. W. (1983) Brain Res. 268, 67-78. [DOI] [PubMed] [Google Scholar]
- 7.Dellovade, T. L., Davis, A. M., Ferguson, C., Sieghart, W., Homanics, G. E. & Tobet, S. A. (2001) J. Neurobiol. 49, 264-276. [DOI] [PubMed] [Google Scholar]
- 8.McCarthy, M. M., Auger, A. P. & Perrot-Sinal, T. S. (2002) Trends Neurosci. 25, 307-312. [DOI] [PubMed] [Google Scholar]
- 9.Luine, V. N., Wu, V., Hoffman, C. S. & Renner, K. J. (1999) Neuroendocrinology 69, 438-445. [DOI] [PubMed] [Google Scholar]
- 10.Mansky, T., Mestres-Ventura, P. & Wuttke, W. (1982) Brain Res. 231, 353-364. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy, M. M., Kaufman, L. C., Brooks, P. J., Pfaff, D. W. & SchwartzGiblin, S. (1995) J. Comp. Neurol. 360, 685-697. [DOI] [PubMed] [Google Scholar]
- 12.Clark, A. S., Myers, M., Robinson, S., Chang, P. & Henderson, L. P. (1998) Proc. R. Soc. Lond B 265, 1853-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng, L. & Chen, G. (2003) Proc. Natl. Acad. Sci. USA 100, 13025-13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen, G., Trombley, P. Q. & van den Pol, A. N. (1996) J. Physiol. 494, 451-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirsch, J. C., Agassandian, C., Merchan-Perez, A., Ben-Ari, Y., DeFelipe, J., Esclapez, M. & Bernard, C. (1999) Nat. Neurosci. 2, 499-500. [DOI] [PubMed] [Google Scholar]
- 16.Kow, L. M. & Pfaff, D. W. (1998) Behav. Brain Res. 92, 169-180. [DOI] [PubMed] [Google Scholar]
- 17.Simerly, R. B. (2002) Annu. Rev. Neurosci. 25, 507-536. [DOI] [PubMed] [Google Scholar]
- 18.Mong, J. A., Glaser, E. & McCarthy, M. M. (1999) J. Neurosci. 19, 1464-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vito, C. C. & Fox, T. O. (1979) Science 204, 517-519. [DOI] [PubMed] [Google Scholar]
- 20.Pfaff, D. & Keiner, M. (1973) J. Comp. Neurol. 151, 121-158. [DOI] [PubMed] [Google Scholar]
- 21.Simerly, R. B. & Young, B. J. (1991) Mol. Endocrinol. 5, 424-432. [DOI] [PubMed] [Google Scholar]
- 22.Shughrue, P. J., Bushnell, C. D. & Dorsa, D. M. (1992) Endocrinology 131, 381-388. [DOI] [PubMed] [Google Scholar]
- 23.Lauber, A. H., Mobbs, C. V., Muramatsu, M. & Pfaff, D. W. (1991) Endocrinology 129, 3180-3186. [DOI] [PubMed] [Google Scholar]
- 24.Kelly, M. J., Lagrange, A. H., Wagner, E. J. & Ronnekleiv, O. K. (1999) Steroids 64, 64-75. [DOI] [PubMed] [Google Scholar]
- 25.Kelly, M. J. & Levin, E. R. (2001) Trends Endocrinol. Metab. 12, 152-156. [DOI] [PubMed] [Google Scholar]
- 26.Vasudevan, N., Kow, L. M. & Pfaff, D. W. (2001) Proc. Natl. Acad. Sci. USA 98, 12267-12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luine, V. N., Grattan, D. R. & Selmanoff, M. (1997) Brain Res. 747, 165-168. [DOI] [PubMed] [Google Scholar]
- 28.Smith, S. T., Brennan, C., Clark, A. S. & Henderson, L. P. (1996) Neuroendocrinology 64, 103-113. [DOI] [PubMed] [Google Scholar]
- 29.Tobet, S. A., Henderson, R. G., Whiting, P. J. & Sieghart, W. (1999) J. Comp. Neurol. 405, 88-98. [DOI] [PubMed] [Google Scholar]
- 30.Davis, A. M., Grattan, D. R., Selmanoff, M. & McCarthy, M. M. (1996) Horm. Behav. 30, 538-552. [DOI] [PubMed] [Google Scholar]
- 31.Davis, A. M., Ward, S. C., Selmanoff, M., Herbison, A. E. & McCarthy, M. M. (1999) Neuroscience 90, 1471-1482. [DOI] [PubMed] [Google Scholar]
- 32.Perrot-Sinal, T. S., Davis, A. M., Gregerson, K. A., Kao, J. P. & McCarthy, M. M. (2001) Endocrinology 142, 2238-2243. [DOI] [PubMed] [Google Scholar]
- 33.Qiu, J., Bosch, M. A., Tobias, S. C., Grandy, D. K., Scanlan, T. S., Ronnekleiv, O. K. & Kelly, M. J. (2003) J. Neurosci. 23, 9529-9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Auger, A. P., Perrot-Sinal, T. S. & McCarthy, M. M. (2001) Proc. Natl. Acad. Sci. USA 98, 8059-8064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obrietan, K. & van den Pol, A. N. (1995) J. Neurosci. 15, 5065-5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Masco, D., Weigel, R. & Carrer, H. F. (1986) Behav. Brain Res. 19, 153-162. [DOI] [PubMed] [Google Scholar]
- 37.McCarthy, M. M., Masters, D. B., Fiber, J. M., Lopez-Colome, A. M., Beyer, C., Komisaruk, B. R. & Feder, H. H. (1991) Neuroendocrinology 53, 473-479. [DOI] [PubMed] [Google Scholar]
- 38.McCarthy, M. M., Malik, K. F. & Feder, H. H. (1990) Brain Res. 507, 40-44. [DOI] [PubMed] [Google Scholar]
- 39.McCarthy, M. M., Masters, D. B., Rimvall, K., Schwartz-Giblin, S. & Pfaff, D. W. (1994) Brain Res. 636, 209-220. [DOI] [PubMed] [Google Scholar]
- 40.Guptarak, J., Selvamani, A. & Uphouse, L. (2004) Brain Res. 1027, 144-150. [DOI] [PubMed] [Google Scholar]
- 41.Kow, L. M. & Pfaff, D. W. (1981) Exp. Brain Res. Suppl 3, 262-273. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.