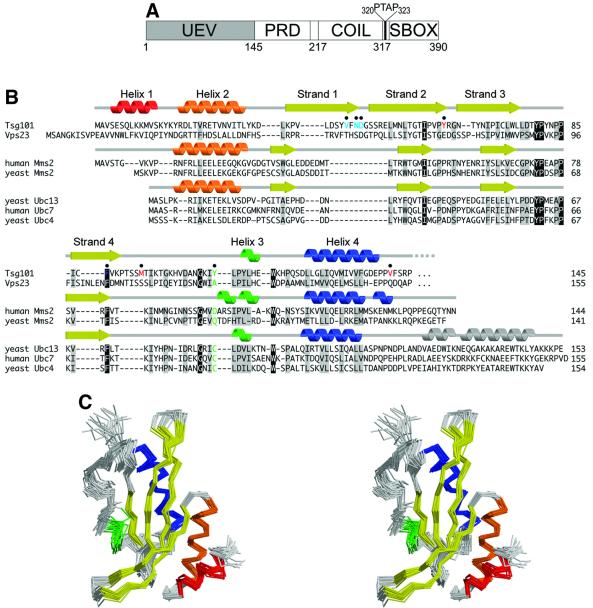
Fig. 1. Sequence and structure of Tsg101. (A) Domain organization of Tsg101, showing the approximate domain boundaries of the UEV domain, proline-rich domain (PRD), putative coiled coil domain (COIL) and ‘steadiness box’ (SBOX) (Feng et al., 2000). The internal PTAP sequence between the coiled-coil domain and the steadiness box is shown explicitly. (B) Structure-based sequence alignment of UEV and E2 proteins. The secondary structures of Tsg101 UEV, uncomplexed human Mms2 (Moraes et al., 2001) and uncomplexed yeast Ubc13 (VanDemark et al., 2001) are shown at the top, middle and bottom, respectively. Conserved residues are shaded gray, identical residues black. The active site cysteine of catalytic E2 enzymes and the equivalent residues in UEV proteins are green. Tsg101 UEV residues that are important for binding PTAP and Ub are colored red and blue, respectively. (C) Stereo view superposition of the final 20 NMR structures of the Tsg101 UEV domain. Secondary structures are colored as in (B).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.