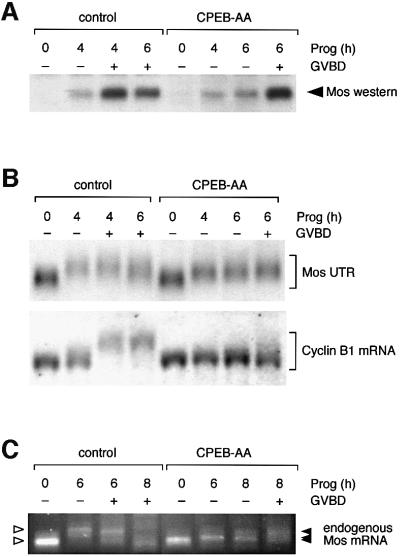
Fig. 1. CPEB-independent control of Mos mRNA translational activation. (A) Western blot of endogenous Mos protein. Immature oocytes were injected with 3–6 ng RNA encoding Xenopus CPEB-AA or water as a negative control, and left for 16 h to express the protein. Oocytes were stimulated by addition of progesterone. Samples were taken for both control and CPEB-AA-treated oocytes when the control oocytes had reached GVBD50 (4 h) and GVBD100 (6 h). Control oocytes were segregated at GVBD50 based on whether they had (+) or had not (–) completed GVBD. Expression of CPEB-AA significantly delayed GVBD but did not prevent low-level Mos protein accumulation prior to GVBD. This effect on Mos accumulation was observed in four independent experiments. At later time points some CPEB-AA-expressing oocytes underwent GVBD. Segregation of these oocytes based on GVBD status revealed that oocytes escaping the CPEB-AA block to maturation then accumulated high levels of Mos protein equivalent to control, post-GVBD, oocytes. (B) Northern blot showing the differential effect of CPEB-AA on polyadenylation of Mos UTR and cyclin B1 mRNA. Total RNA was prepared from the same oocyte samples described in (A) and analyzed for the polyadenylation status of either the co-injected wild-type terminal 48-nt Mos 3′ UTR (Mos UTR) or the endogenous cyclin B1 mRNA. Polyadenylation (retarded mobility) of the Mos UTR was observed in CPEB-AA-expressing oocytes. This experiment was repeated three times with similar results. In contrast, cyclin B1 polyadenylation was inhibited in CPEB-AA-expressing oocytes. (C) Polyadenylation of endogenous Mos mRNA analyzed by RT–PCR. In this experiment GVBD50 was at 6 h and GVBD100 was at 8 h. Polyadenylation (retarded mobility) of the Mos mRNA was observed in CPEB-AA-expressing oocytes following progesterone stimulation. A representative experiment is shown.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.