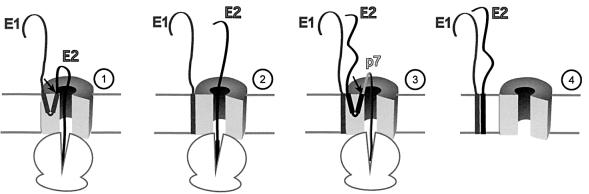
Fig. 8. Model of the behaviour of the TM domains of HCV envelope glycoproteins E1 and E2 during the early steps of their biogenesis. (1) The N-terminus of E1 is translocated into the lumen of the ER and also orients the N-terminus of the TM domain of E1 toward the ER lumen. The C-terminal half of the TM domain of E1 is the signal sequence of E2 and has its C-terminus oriented toward the lumen of the ER to allow the translocation of E2. At this step and before signal sequence cleavage, the TM domain of E1 forms a hairpin structure that is likely to be located in the environment of the translocon. (2) After signal sequence cleavage between E1 and E2, the signal sequence present in the C-terminal half of the TM domain of E1 is reoriented toward the cytosol and E1 probably remains very close to the translocon. (3) As shown for the TM domain of E1, the TM domain of E2 transiently adopts a hairpin structure to allow the translocation of p7. (4) After signal sequence cleavage between E2 and p7, the signal sequence present in the C-terminal half of the TM domain of E2 is reoriented toward the cytosol, the TM domains of E1 and E2 interact and move in the lipid bilayer. It is worth noting that, in the context of HCV polyprotein, the cleavage between E2 and p7 is delayed (T½ ≈15 min) (Dubuisson, 2000). Owing to the absence of experimental data on its topology, p7 is not included in step 4.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.