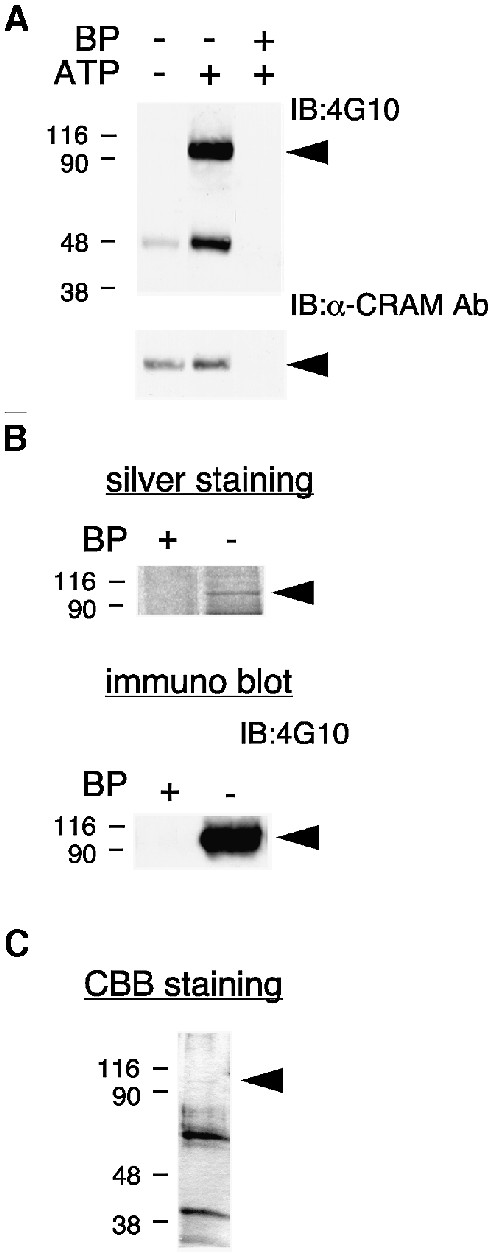
Fig. 1. Isolation of CRAM-associated PTK. (A) Association of PTK activity in the CRMP–CRAM complex. Rat brain lysates were immunoprecipitated with anti-CRAM Ab in the presence or absence of blocking peptide (BP) derived from CRAM protein. Immuno precipitates were incubated with the kinase reaction mixture in the presence or absence of ATP for tyrosine phosphorylation, and then immunoblotted with anti-phosphotyrosine Ab (4G10) or anti-CRAM Ab. (B) Silver staining of purified 95 kDa protein. CRAM-associated proteins were purified from rat brain lysates in the presence or absence of blocking peptide. The 95 kDa protein in the final step was analyzed by silver staining or immunoblotting with 4G10. (C) Large-scale purification of 95 kDa protein. Purified 95 kDa protein in the final step was analyzed by Coomassie Brilliant Blue (CBB) staining. The position of 95 kDa protein is indicated by the arrowheads.
