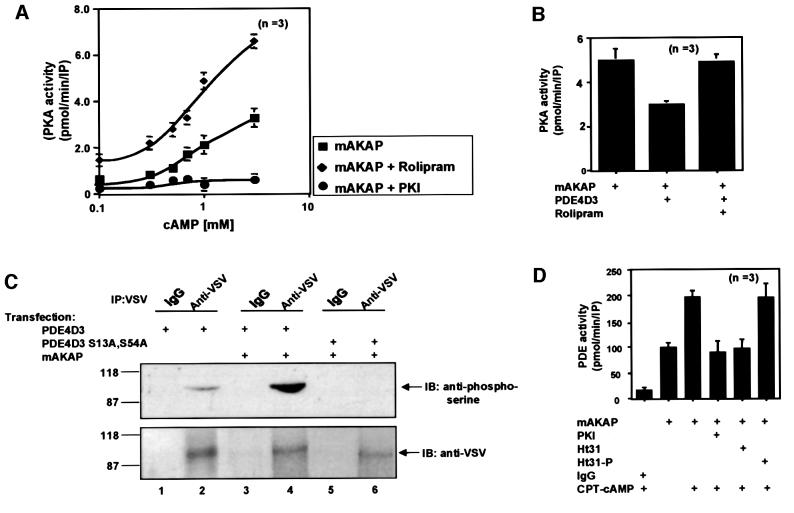
Fig. 5. mAKAP assembles a PDE/PKA negative feedback loop. The functional consequences of PDE4D3 localization through association with mAKAP were assessed on PKA activity. (A) The mAKAP signaling complex was immunoprecipitated from rat heart extracts using antibodies against the mouse anchoring protein. PKA activity (squares) was measured over a range of cAMP concentrations (0.1–10 mM) as described in Materials and methods, upon inhibition of PDE4D3 by rolipram (diamonds) and in the presence of the PKI 5–24 peptide (circles). The specific activity of the PKA catalytic subunit is presented as pmol/min/IP. The averaged data from three experiments are presented. (B) Recombinant myc-tagged mAKAP was expressed in HEK293 cells in the absence or presence of PDE-4D3 (indicated below each lane). Anti-Myc antibodies were used to isolate immune complexes and PKA activity was measured in the presence of 1 mM cAMP. The specific activity of the PKA catalytic subunit is presented as pmol/min/IP. The averaged data from three experiments are presented. Rolipram (10 µM) was used to selectively inhibit PDE activity. (C) Expression vectors encoding VSV-tagged PDE4D3 (lanes 1–4) or the phosphorylation site mutant VSV-tagged PDE4D3 S13A,S54A (lanes 5 and 6) were co-expressed with myc-tagged mAKAP in HEK293 (lanes 2–6). The transfected plasmids are indicated above each lane. The cells were incubated with 1 mM CPT-cAMP for 15 min before immunoprecipitation with anti-VSV antibodies or control IgG. The immune complexes were separated by gel electrophoresis on a 7.5% SDS–polyacrylamide gel and electrotransferred to nitrocellulose membranes. Phosphorylation of PDE4D3 was assessed by immunoblotting (IB) using a polyclonal phosphoserine-specific antibody (top panel). Equal loading of PDE4D3 was confirmed by reprobing the blot with VSV-specific monoclonal antibody (bottom panel). Detection of the signals was by chemiluminescence. Molecular weight markers and the migration position of PDE are indicated. This figure is representative of three independent experiments. (D) In reciprocal experiments, the functional consequences of PKA anchoring were assessed on PDE4D3 activity. The mAKAP signaling complex was immunoprecipitated from rat heart extracts using antibodies against the rat anchoring protein. After a 16 h incubation, the immunoprecipitates were incubated in the presence or absence of CPT-cAMP for 30 min (indicated below). PDE4 activity was then measured under conditions where PKA activity was inhibited (PKI), in the presence of a PKA anchoring inhibitor peptide (Ht31) or a control peptide (Ht31p). PDE activity was presented as pmol/min/IP. The data averaged from three experiments are presented.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.