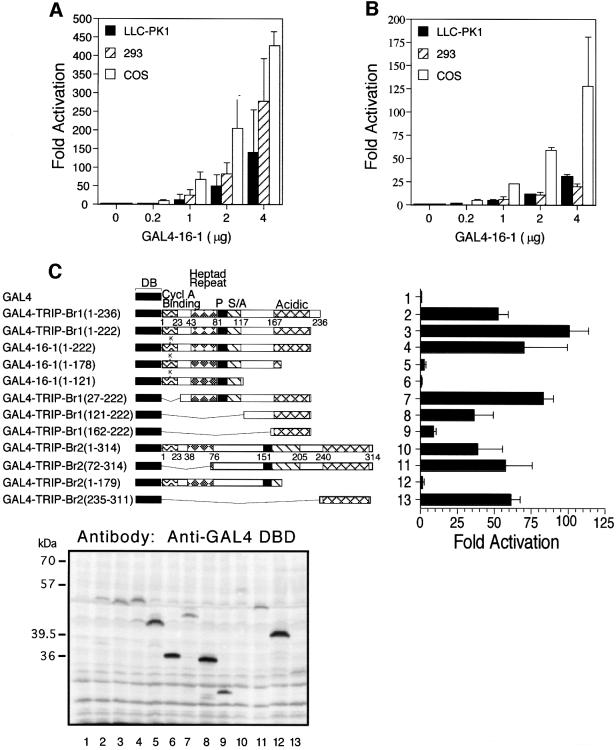
Fig. 6. Transcriptional activation by TRIP-Br proteins recruited to a heterologous promoter. (A) Cell lines were transiently transfected with the indicated amounts of the SV40 expression vector pBXG1/16-1 encoding the entire 16-1 insert fused in-frame with GAL4-DB, 1.5 µg of the luciferase minimal reporter plasmid pG5-GL3 bearing five copies of the GAL4 DNA-binding sequence followed by the E1b TATA box, and 0.1 µg of pCMV/β-galactosidase expression vector. Fold activation refers to luciferase activity normalized to β-galactosidase activity, and is expressed relative to the activity observed with transfection of the reporter alone. Values represent the average ± standard deviation of three or four independent experiments. (B) Transient transfections were performed as in (A) except that the upstream SV40 enhancer reporter plasmid pG5 (SV)-GL3 was used in 293 cells, while the downstream SV40 enhancer reporter plasmid pG5-GL3 (SV) was used in COS and LLC-PK1. (C) Schematic of various GAL4/TRIP-Br deletion mutants (left) and their respective abilities to activate a heterologous minimal promoter (right) are shown. Transient transfections were performed as in (A) with 0.5 µg of each expression construct. Western blot analysis (below) of 50 µg of total cell lysate from each transfection was performed with anti-GAL4 DB mouse monoclonal antibody (Santa Cruz). The GAL4/16-1 (1–222) fusion protein (lane 4) migrates more slowly than the GAL4/TRIP-Br1 (1–222) fusion protein (lane 3), due to the presence of a ‘linker’ (LRSDWPRVICR) encoded by the short 5′-UTR of the original 16-1 clone.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.