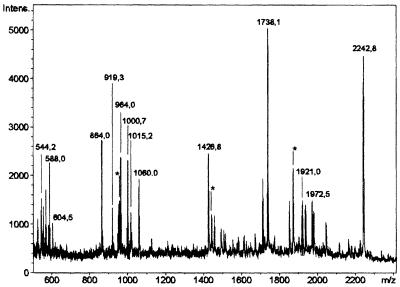
Fig. 2. Mass spectrometric analysis of a tryptic digest obtained from the ADP-ribosylated ∼53 kDa mitochondrial protein. The incubation and separation of the modified protein were conducted as described in the legend to Figure 1. Following SDS–PAGE, the protein band containing the radioactive label was subjected to ‘in-gel’ digestion with trypsin. The mass spectrum of the resulting peptides was obtained by MALDI-TOF spectrometry. Only masses corresponding to internal peptides of GDH are indicated, assuming a maximum of one missed cleavage site per peptide. The asterisks indicate peaks corresponding to GDH peptide masses, considering more than one missed cleavage site or oxidized methionine residues.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.