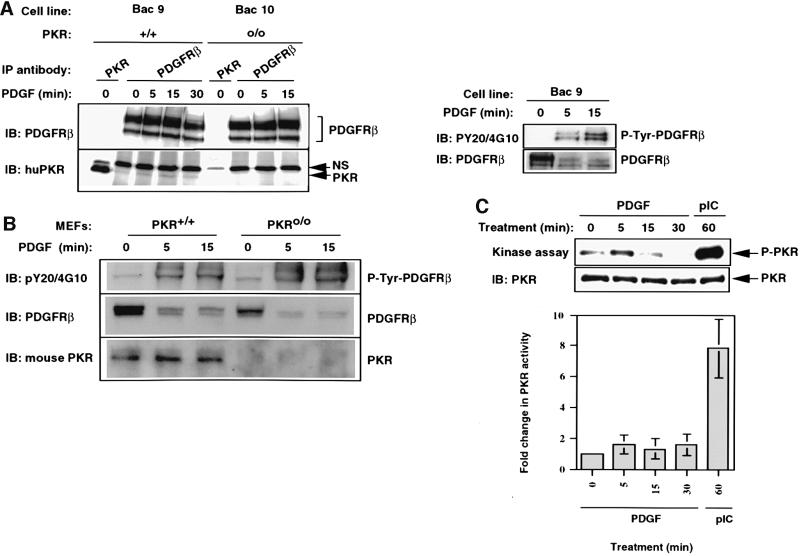
Fig. 3. PKR associates with the PDGF β-receptor in a constitutive manner. (A) Bac9 and Bac10 cells were serum starved overnight and then treated with PDGF (20 ng/ml) as described in Materials and methods. Whole-cell extracts (1 mg, left side; 400 µg, right side) were immunoprecipitated with anti-PDGFR β antibody (pAb). Immunoprecipitates were immunoblotted with PDGFR antibody (top panel, left side) or PKR antibody (BCI, lower panel, left side). The figure on the right shows that the receptor was tyrosine phosphorylated in response to PDGF. The membrane was first probed for phosphotyrosines (PY20 and 4G10 polyclonal) before being stripped and re-probed for total PDGFR β protein. NS refers to a non-specific cross-reacting protein band. (B) PKR+/+ and PKRo/o MEFs were serum starved for 18 h and treated with PDGF (20 ng/ml) for the indicated times. Whole-cell extracts (300 µg) were immunoprecipitated with anti-PDGFR β antibody and subjected to SDS–PAGE analysis. The top portion of the blot was probed initially for tyrosine-phosphorylated receptor (top panel) and then stripped and re-probed for total receptor (middle panel). The lower half of the blot was immunoprobed for PKR using a mouse polyclonal antibody raised against mouse PKR (lower panel). (C) Mouse PKR-null cells reconstituted for PKR by stable transfection of a BAC clone carrying human PKR gene (Bac9) were treated with PDGF (20 ng/ml) or pIC (100 µg/ml) following overnight starvation. In vitro kinase assays were performed on immunoprecipitated PKR (from 150 µg of extract) as described in Materials and methods. Following autoradiography (top panel), the membrane was immunoprobed for the presence of PKR protein in each IP (lower panel). The graph reflects the mean phosphorimager quantitation of autophosphorylated PKR from three separate experiments, each normalized for the amount of immunoprecipitated PKR.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.