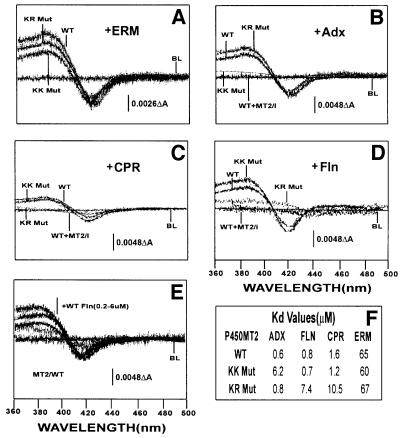
Fig. 5. Extent of binding of wild-type and mutated P450MT2 to various electron transfer proteins by spectral shift measurements. Shifts in the spin state of bacterially expressed and purified wild-type (WT), KK Mut and KR Mut P450MT2 by added substrate or various electron transfer proteins were measured spectrophotometrically as described in Materials and methods. Effects of (A) erythromycin (ERM, 0.5 mM), (B) Adx (6 µM), (C) CPR (4 µM) and (D) Fln (6 µM). In (B–D), the effect of a 70-fold molar excess of P450MT2/I peptide was used as a positive control. (E) The effects of increasing amounts (0.2–0.6 µM) of Fln. The table in (F) shows the Kd for P450MT2 binding to various electron transfer proteins. The Kd value for Fln was calculated from ΔOD in (E), and the values for Adx and CPR were as reported before (Anandatheerthavarada et al., 1998).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.