Abstract
Ovarian cancer (OVCA) is third most lethal gynecologic cancers and acquired chemoresistance is the key link in the high mortality rate of OVCA patients. Currently, there are no reliable methods to predict chemoresistance in OVCA. In our study, we identify genes, pathways and networks altered by DNA methylation in high-grade serous ovarian carcinoma (HGSC) cells that are associated with chemoresistance and prognosis of HGSC patients. We performed methylome-wide profiling using Illumina Infinium MethylationEPIC BeadChip (HM850K) methylation array on a set of HGSC chemoresistant and chemosensitive cell lines. Differentially Methylated CpG Probes (DMPs) were identified between the resistant and sensitive groups in HGSC. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) over-representation analyses were conducted to identify both common and unique pathways between resistant and sensitive cells. While the HM850K array was used for the discovery phase to identify differentially methylated probes and regions in HGSC cell lines, the publicly available The Cancer Genome Atlas ovarian cancer (TCGA-OV) dataset generated using the Illumina Infinium HumanMethylation27 BeadChip (27 K array) methylation array served as an independent validation cohort for downstream survival and drug sensitivity analyses. Machine learning methods were applied to our dataset to predict drug sensitivity in the TCGA-OV cohort and to investigate associations with overall survival and progression-free survival. Kaplan-Meier analysis was performed to assess the relationship between differentially methylated genes and patient survival outcomes. The overlapping CpG probes shared between the two Illumina platforms were used for machine learning and survival analyses. Data visualization was performed using various R/Bioconductor packages. Our analysis identified a total of 3,641 DMPs spanning 1,617 differentially methylated genes between chemoresistant and sensitive HGSC cells, whereas 80% of them were hypermethylated CpG sites associated with HGSC resistant cells. Approximately half of the DMPs were distributed on chromosomes 1–3, 6, 11–12 and 17 and top identified hypermethylated CpGs were cg21226224 (SOX17, ∆β = 79%, adj.P = 7.73E-03), cg02538901 (ATP1A1, ∆β = 75%, adj.P = 7.6E-03), and cg17032184 (CD58, ∆β = 64%, adj.P = 4.39E-02). Machine learning analysis identified significant association of global hypermethylation in the HGSC chemoresistant cells with poor overall and progression-free survival of HGSC patients. Further analysis identified four differentially methylated genes (CD58, SOX17, FOXA1, ETV1) that were also positively associated with poor prognosis of HGSC OC patients. Functional enrichment analysis showed enrichment of several cancer-related pathways, including phosphatidylinositol signaling, homologous recombination and ECM-receptor interaction pathways. This study supplements the current knowledge of the underlying mechanism behind acquired chemoresistance in OVCA. Four differentially methylated genes identified in this study may have the potential to serve as promising epigenetic clinical biomarkers for HGSC chemotherapy resistance.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-20827-8.
Subject terms: Epigenetics, Epigenomics, Computational biology and bioinformatics, Systems biology, Biomarkers, Molecular medicine, Oncology, Cancer, Gynaecological cancer, Tumour biomarkers
Introduction
Ovarian cancer (OVCA) is the third most lethal cancer of the female reproductive system, worldwide1. Despite the diagnostic and therapeutic advancement, the 5-year survival rate for OC remains between 20 and 40% for patients with late stage disease2. Epithelial ovarian cancer (EOC) is well characterized and accounts for ~ 90% of all OC cases3. EOC is further sub-classified into five histotypes among which high-grade serous ovarian carcinoma (HGSC) is the most prevalent subtype4. Standard care of treatment for HGSC is surgical debulking combined with platinum and taxane-based chemotherapy5. Although, this treatment combination initially shows promising clinical response in HGSC patient, 70–80% of responding HGSC patients relapse and develop resistance to chemotherapy which is the key link in the high mortality rate of HGSC patients6,7. The molecular mechanisms of HGSC chemoresistance can be heterogeneous and complex including abnormalities in multiple genetic and epigenetic factors8–13. Detailed characterization of molecular mechanisms associated with HGSC chemoresistance are needed for the development of improved therapies and better prognosis of HGSC patients.
Epigenetic mechanisms regulate and ensure normal genome functioning, whereas epigenetic aberrations often result in disease development such as cancer14,15. DNA methylation is one of the most widely studied epigenetic mechanism associated with gene regulation in chemoresistance development16. Aberrant methylation in tumor suppressor genes and oncogenes are two common epigenetic modifications occurring in all cancers, including ovarian cancer15,17. Pre-clinical and clinical studies have also demonstrated the effect of DNA methylation in OC chemoresistance18–20. Multiple genes including hMSH221, MSXI18, ABCB122, ZNF67123, and many others have been identified whose aberrant promoter methylation status were associated with OC chemoresistance. DNA methylation changes in these genes resulted in dysregulated drug export, DNA mismatch repair and apoptotic pathways which lead to chemoresistance18,21–23. Despite the enormous studies on OC chemoresistance, there is no reliable marker to enable clinicians predict chemoresistance pre- and post-treatment. DNA methylation signatures could therefore be a promising prognostic biomarker because of their reversible nature, chemical stability, as well as less-invasive and quantitative detection24.
The overall objective of this study was to identify genes, pathways and networks altered by DNA methylation in HGSC cells that are associated with chemoresistance and prognosis of HGSC patients. We performed methylome-wide profiling using HM850K methylation array with the highest methylome coverage on a set of HGSC chemoresistant and chemosensitive cell lines as well as other histological subtypes. Further, we used multiple bioinformatics tools to make comparisons of pathways and networks associated with differentially methylated genes generated from HGSC chemo-sensitive and resistant cells. Here, we present an extensive global methylation of HGSC chemoresistant and chemosensitive cells and how that relates to patient outcomes.
Materials and methods
Cell lines and cell culture
Chemosensitive and chemoresistant OC cell lines of HGSC histologic subtypes were used in this study. HGSC cell lines (chemosensitive; TOV3133G, TOV3041G, chemoresistant; TOV3133R, OV90, TOV3291G, 433OVCA) were kindly provided by Dr. Anne-Marie Mes-Masson (Centre de recherche du Centre hospitalier de l’Université de Montréal (CRCHUM), Montreal, QC, Canada). The characteristics of the cells are as follows: TOV3133G (nonsense-sensitive), TOV3133R (p53 mutant; Gln192Ter-resistant), TOV3041G (nonsense-sensitive), OV90 (p53 mutant; Ser215Arg-resistant)25–27. All the cell lines were validated at SickKids Centre for Applied Genomics Genetic Analysis Facility. Endometrioid and HGSC cell lines were cultured in RPMI-1640 and OSE media respectively, with 10% fetal bovine serum (FBS) at 37 °C in humidified 5% CO2 incubator.
Samples processing
Genomic DNA was extracted from cells using AllPrep DNA/RNA mini kit and DNeasy Blood & Tissue Kit (Qiagen, Toronto, ON, Canada) according to the manufacturer’s protocol. The extracted DNA concentrations were quantified using Qubit 4 (Thermo Fisher Scientific, Waltham, MA, USA) and final DNA concentration was adjusted to 20 ng/µL. For methylome-wide profiling, 500 ng of extracted DNA was bisulfite converted using EZ DNA methylation kit (Zymo Research, Irvine, CA, USA). 250 ng of bisulfite converted DNA was analyzed using Infinium MethylationEPIC BeadChip microarray (HM850K; Illumina (Illumina Inc., San Diego, CA, USA28, which allows to determine genome-wide methylation profile at single-base resolution covering more than 850,000 CpG sites29. Methylome-wide data was analyzed by Epigenomics and Mechanisms Branch at the International Agency for Research of Cancer30.
Preprocessing of EPIC DNA methylome-wide array
All analyses were conducted in R software (version 4.3.3). Raw data files (.idat) from the HM850K (~ 850 K CpG probes) were preprocessed utilizing the R/Bioconductor package minfi (version 1.48.0)31. Probes were excluded if they met any of the following criteria: a detection p-value > 0.01 in at least one sample, localization on the X and Y chromosomes, presence within SNP loci, cross-reactivity (as determined by the R package maxprobes, version 0.0.2), or fewer than three bead counts. This filtering process yielded 752,914 CpG probes for subsequent analysis32. M-values were derived from β-values following normalization, which was carried out using the Noob and Quantile normalization methods available in minfi. To further ensure data integrity, principal component analysis (PCA) was performed to evaluate potential technical variability and identify outliers, complemented by quality control assessments through the estimation of beta distributions.
Differential methylation analysis
Differentially Methylated CpG Probes (DMPs) were identified using the R/Bioconductor package limma (version 3.58.1) through linear model fitting between the resistant and sensitive groups in HGSC33. M-values were employed in the analysis, and the false discovery rate (FDR) method was applied to adjust p-values for multiple testing. DMPs with an FDR-adjusted p-value < 0.05 and a delta beta change ≥ 0.2 were considered statistically significant. This cutoff was chosen based on established conventions in the field, balancing biological relevance with control of technical variability, while ensuring high-confidence detection of differential methylation34. Differentially Methylated Regions (DMRs) with an HMFDR value < 0.05 and a mean delta beta difference ≥ 0.2 were also deemed significant. DMRs were detected using the R/Bioconductor package DMRcate (version 2.16.1)35. This tool defines a DMR as a genomic region containing multiple adjacent CpG probes that show consistent differential methylation between comparison groups. Specifically, DMRcate first calculates smoothed t-statistics across genomic regions by aggregating nearby DMPs that are within a user-defined distance (default = 1,000 bp). Regions that contain at least two or more significant DMPs (based on FDR < 0.05 and consistent directionality of methylation change) are merged into a single DMR. Each DMR is then assigned a combined test statistic and average delta beta value to reflect the overall regional methylation change. Thus, although DMRs may encompass individual significant DMPs, the overall significance of a DMR depends on the combined methylation difference across the region and the aggregated statistical significance, and not all DMP clusters necessarily form significant DMRs, as shown in Fig. 2.
Fig. 2.
Distribution of DMPs and DMRs among all autosomes.
The data were annotated using the Illumina EPIC annotation (R/Bioconductor package IlluminaHumanMethylationEPICanno.ilm10b4.hg19, version 0.6.0) and further annotated by CpG island relationships and genomic regions using the R/Bioconductor package annotate (version 1.28.0)36,37. Furthermore, tumor suppressors and oncogenes that overlap with significant DMPs, according to the cancer gene list in OncoKB, were interrogated in the Infinium HumanMethylation27 BeadChip (27 K array; Illumina (Illumina Inc., San Diego, CA, USA) ovarian cancer data from The Cancer Genome Atlas (TCGA-OV)38,39. The top ten significantly methylated genes (five hypermethylated tumor suppressors and five hypomethylated oncogenes) were selected to assess their contribution to overall survival in ovarian cancer using Kaplan-Meier (KM) survival analysis40. TCGA-OV data were split into hypomethylated and hypermethylated groups according to the optimal cut-off approach, and the survival significance between groups was accepted as p-value < 0.05. Only the genes that were also present in the TCGA-OV dataset were included in the analysis.
Data visualization
Methylation results were further visualized using heatmaps generated by the R/Bioconductor package Complex Heatmap (version 2.15.4) for DMPs and DMRs, employing the Euclidean method for hierarchical clustering41. Volcano plots were created using the R/Bioconductor package Enhanced Volcano (version 1.20.0), highlighting the corresponding genes in significant DMPs and DMRs42. Genes overlapping multiple CpG probes were summarized by their mean delta beta change. Circos plots were produced using the R/Bioconductor package circlize (version 0.4.15) by sub-setting hypermethylated and hypomethylated regions in both DMPs and DMRs43. Tumor suppressors and oncogenes in these regions were selected for visualization using the cancer gene list from OncoKB44. Stacked bar charts, regional plots, and enrichment charts were generated using the R package ggplot2 (version 3.5.0)45. Network plots for enriched terms were visualized using the R/Bioconductor package enrichplot (version 1.22.0)46. Kaplan-Meier survival plots were generated using the R package survminer (version 0.4.9)47. Raw p-values were calculated by default using the ggsurvplot function. Survival differences were considered significant at a raw p-value < 0.05. For analyses involving multiple genes (Fig. 3B), FDR-adjusted p-values were subsequently calculated and are provided in Supplementary File Table-6. For the other survival analyses based on machine learning and global methylation, we reported raw p-values only.
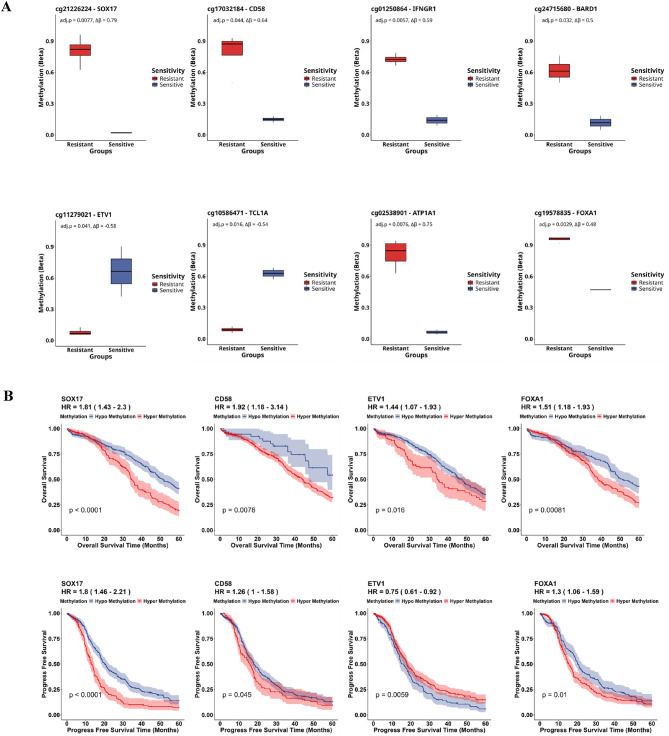
Fig. 3.
Differential methylation identifies potential signatures for HGSC chemoresistance. (A) Box plots representing methylation changes in significant probes/genes enriched in cancer pathways along with their ∆β change and FDR adjusted p-values. Blue boxes represent HGSC sensitive samples, while red boxes represent HGSC resistant samples. (B) TCGA (TCGA-OV) was analyzed to find the correlation of differentially methylated genes to overall survival and progression-free survival in ovarian cancer patients using Kaplan-Meier (KM) survival analysis. (C) Kaplan-Meier plot demonstrating the overall survival risk and Progression free survival risk of resistant (red) and sensitive (blue) samples in OV-TCGA data based on the drug sensitivity prediction from our dataset of 14 samples. (D) Kaplan-Meier plot demonstrating overall survival, progression free survival and chemoresistance prediction of hypomethylation (blue) and hypermethylation (red) from public dataset.
Functional enrichment analysis
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) over-representation analyses were conducted based on active subnetworks using the R package pathfindR (version 2.4.1)48–51. Analyses were conducted using the full set of significant genes from each, with hypo and hypermethylated representative probes combined into a single list. A gene was classified as hypomethylated or hypermethylated based on the average methylation change (including sign) of its associated significant probes, all of which had FDR < 0.05.
Enrichment analyses were first performed separately for DMPs and DMRs. For GO and KEGG enrichment visualizations (Fig. 4A) (Supplementary File Tables 3, 4 and 5), we primarily show the results from the DMP-based analysis. Pathways with a fold enrichment value ≥ 1 and FDR-adjusted p-value < 0.05 were considered significant then the top 25 significantly enriched pathways from both GO and KEGG analyses are shown in the main figures to highlight key biological processes.
Fig. 4.
Identification of signalling pathways relevant to methylation signature of HGSC chemoresistance. (A) KEGG and GO enrichment analyses were conducted on significant genes associated with DMPs. KEGG pathway enrichment analysis of differentially methylated genes was performed using the pathfindR package in R. The x-axis denotes the fold enrichment (> 1) while the y-axis represents the KEGG signaling pathways with color representing the –log10 FDR adjusted p-value. In the GO enrichment plot, the vertical axis indicates the GO ontologies by biological process (BP-orange), cellular component (CC-blue), and molecular function (MF-green), while the horizontal axis shows the –log10(FDR). (B) Network plot illustrates the relationships between selected significantly enriched pathways, with nodes representing pathways and edges indicating interactions. Pathways are grouped into clusters using kmeans in clusterProfiler package by selecting four representative words from each pathway of a cluster (pink, green, blue, and purple). Pathways are annotated with significantly enriched hypo (blue) and hyper (red) methylated genes in the related pie charts according to their counts. The legend with circles represents the size of the pie based on overall enriched gene sets.
Additionally, we applied a combined analysis using the combine_pathfindR_results function to identify pathways (KEGG only) commonly enriched in both DMP and DMR datasets. This integrated result was used as a pathway selection criterion to highlight the most cancer-related pathways consistently overrepresented across both types of methylation changes (Supplementary File Table-5).
To explore methylation directionality within the enriched pathways that derived from combined analysis, we applied the compareCluster function from the clusterProfiler package (version 4.10.1)52 (Fig. 4B).
Survival analysis using machine learning
Machine learning methods were employed to predict drug sensitivity in independent OC methylation data from the 27 K array in TCGA (TCGA-OV), as well as to investigate the overall survival and progression-free survival in relation to drug response. To ensure compatibility between our discovery dataset (HM850K array) and the TCGA-OV validation dataset (27 K array, 591 samples), we identified and retained only the overlapping CpG probes (25,098 probes) shared between both platforms. Missing beta values in the TCGA-OV data were removed, resulting in 18,133 overlapping probes, after which quantile normalization was applied. OurHM850K data, consisting of 14 samples (9 resistant and 5 sensitive), was filtered by these overlapping probes and subsequently used for feature selection. The Recursive Feature Elimination (RFE) method was applied to identify significant features within the HM850K, utilizing the R package caret (version 6.0–94)53,54. Furthermore, our HM850K data with the 100 selected significant features from the RFE analysis were used to build a training model through the Elastic Net regression-based prediction method, implemented in the R package glmnet (version 4.1-8), to predict drug sensitivity in the TCGA-OV dataset55. Missing survival data were excluded, and Kaplan-Meier survival analysis was conducted with data censored at 60 months56. The difference in survival probability between the resistant and sensitive groups was considered significant at a raw p-value < 0.05.
Results
Elevated genome-wide DNA methylation status is associated with resistance in HGSC
To investigate the differential methylation between chemoresistant and chemosensitive cells, we performed a global methylation analysis using Infinium MethylationEPIC BeadChip microarray (HM850K; Illumina28 to quantitatively analyze over 850,000 CpG position across the genome in six HGSC histotypes.
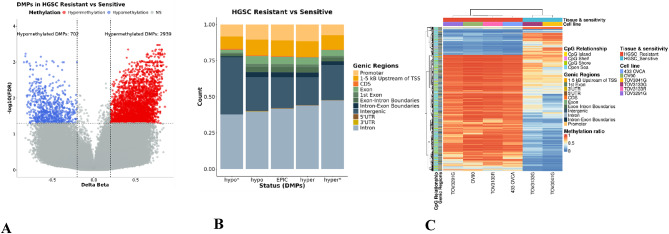
The comparison between HGSC chemoresistant and sensitive cells revealed significant differences in global methylation. A total of 3,641 DMPs spanning 1,617 differentially methylated genes were identified with cutoff criteria ∆β > 0.2 and FDR < 0.05 between chemoresistant and sensitive cells. Among the 3,641 DMPs identified, 2,939 (80%) were hypermethylated CpG sites associated with HGSC resistant cells, with the other 20% associated with chemosensitive cells (Figure 1 A). Interrogation of the enrichment distribution of CpG regulatory regions revealed a significant enrichment in HGSC resistant cells of the hypermethylated DMPs in regions 1-5Kb upstream of the transcription start site and in promoter regions (Fig. 1B and C) (Supplementary File Table-1). To account for potential platform design bias, we compared the distribution of significant DMPs to the background distribution of all probes on the EPIC array (“EPIC” reference group in Figure 1B). Given that the EPIC array is enriched for promoter regions, CpG islands, and regulatory elements, the observed enrichment of DMPs in specific genomic features must be interpreted relative to this array design bias. Out of the 472 chemoresistant-associated DMRs that passed the cutoff criteria ∆β > 0.2 and FDR < 0.05, 451 (95%) regions were hypermethylated compared to sensitive cells (Supplementary Figure 1). After observing a global DNA hypermethylation in HGSC resistant cells, we extended our investigations to determine the distribution of DMPs on the 22 autosomal chromosomes. Our analysis indicated that ~50% of DMPs were distributed on chromosomes 1-3, 6, 11-12 and 17 with multiple known tumor suppressor genes (e.g. ATP1A1, CD58, SOX17, IFNGR1) and oncogenes (e.g. SF3B2, BCL2, SOS1, FURIN) being differentially methylated in the promoter and 1-5 kb TSS region of HGSC resistant cells. Top identified hypermethylated CpGs were cg21226224 (SOX17, ∆β = 79%, adj.P=7.73E-03), cg02538901 (ATP1A1, ∆β = 75%, adj.P=7.6E-03), and cg17032184 (CD58, ∆β = 64%, adj.P=4.39E-02) (Figure 2) (Supplementary File Table-2). These findings indicate that elevated genome-wide methylation is associated with HGSC chemoresistance.
Fig. 1.
Higher level of global methylation observed is in HGSC chemoresistant cells compared to sensitive cells. (A) Volcano plot shows the total of significantly hyper, and hypo methylated DMPs (3641). Red and blue dots represent hypermethylated and hypomethylated DMPs, respectively. Grey dots represent non-significant DMPs. (B) Stacked bar plot illustrates the distribution of DMPs across various genic regions in resistant HGSC compared to sensitive HGSC. The x-axis represents the DMP status, categorized into hypo*, hypo, EPIC, hyper, and hyper*, while the y-axis denotes the count of DMPs. The Asterisk (*) symbol represents significant DMPs with HMFDR < 0.05 and delta beta change ≥ 0.2. Each bar represents the proportion of DMPs found in annotated genic regions, while the “EPIC” reference group represents the background distribution of all probes on the HM850K array. (C) Heatmap illustrates the overall DMPs in HGSC resistant vs. sensitive across various genomic regions annotated with cell lines and drug sensitivity. The rows represent CpG sites categorized by their relationship to CpG islands (CpG island, CpG shelf, CpG shore, open sea) and their genic regions. The columns represent the drug sensitivity (resistant in red, sensitive in blue) corresponding to cell lines. The color gradient from blue to red indicates the methylation ratio, with blue representing lower methylation and red representing higher methylation. The dendrograms show hierarchical clustering using Euclidean distance.
The circos plot illustrates the landscape of genomic methylation changes across autosomal chromosomes, focusing on significant (FDR < 0.05 and Δβ ≥ 0.2) tumor suppressor genes and oncogenes. From the outermost to the innermost ring: the first ring displays human chromosomes annotated with genes. Genes positioned on the outer side of the chromosome track correspond to genes associated with hypermethylated (red) and hypomethylated (blue) differentially methylated probes (DMPs). Genes on the inner side of the chromosome track correspond to genes overlapping with hypermethylated (red) and hypomethylated (blue) differentially methylated regions (DMRs). Lines extending from the gene names point to their corresponding genomic locations on the chromosomes. The second ring displays individual DMPs as red (hypermethylated) and blue (hypomethylated) dots. The third and fourth rings show density plots of DMRs, with red peaks indicating hypermethylated regions and blue peaks indicating hypomethylated regions. The innermost ring highlights the locations of CpG islands in green.
Global methylation analysis identified novel DNA methylation markers of HGSC chemoresistance
We extended our analysis to screen the hyper/hypomethylated CpG sites within the promoter region and 1-5Kb from TSS, both of which are strongly associated with gene expression regulation by DNA methylation57. We identified four hypermethylated tumor suppressor genes (SOX17, CD58, IFNGR1, BARD1), two hypomethylated oncogenes (TCL1A, ETV1) and two hypermethylated proto-oncogenes (FOXA1, ATP1A1) that present as novel DNA methylation signatures associated with HGSC chemoresistance (Fig. 3A). We further investigated the association between the above-mentioned signatures and patient’s outcome. Kaplan-Meier analysis using data from 27 K array in TCGA-OV revealed that hypermethylation of CD58, SOX17 and FOXA1, and hypomethylation of ETV1 was positively associated with poor prognosis of HGSC OC patients (Fig. 3B). We investigated the overall survival (OS) and progression-free survival (PFS) in relation to drug response by applying machine learning on our data and employed it in independent ovarian cancer methylation data from the 27 K array in TCGA-OV. With our machine learning approach, we observed that global hypermethylation in the HGSC chemoresistant cells is significantly associated with poor overall and progression-free survival of HGSC patients (Fig. 3C). The difference in survival probability between the resistant and sensitive groups was considered significant at a p-value < 0.05 (Fig. 3C). Further, we also demonstrated that patients with hypomethylated genes are more responsive to chemotherapy and have prolonged survival relative to patients with hypermethylated genes (Fig. 3D).
Functional enrichment analysis identified signaling pathways relevant to methylation signature of HGSC chemoresistance
To understand the overall functional significance of the 3,641 DMPs identified including the 1,617 genes from our analysis, we performed gene ontology (GO) enrichment analysis with three annotations including biological process, cellular component and molecular functions. The functional enrichment analysis indicated that the most significantly enriched biological processes are regulation of DNA-templated transcription followed by the regulation of small GTPase mediated signal transduction. Similarly, KEGG (Kyoto Encyclopedia and Genomes) pathways enrichment analysis showed most enrichment in several cancer-related pathways, including phosphatidylinositol signaling, homologous recombination and ECM-receptor interaction pathways (Fig. 4A). This enrichment was evidenced by the hypomethylation of key genes such as BRCA1, LAMA3, MSH2, and TCL1A involved in cancer progression and chemoresistance21,58–60.
To identify both common and unique pathways, we integrated enrichment results from DMPs and DMRs and investigated the interaction between hypo- and hyper-methylated genes within the significantly enriched common pathways. Integrated enrichment analysis further confirmed the enrichment of hypermethylated TSGs that were linked with several cancer and chemoresistance related pathways including platinum drug resistance, PI3K-Akt signalling, Wnt signalling, TNF signalling, and p53 signalling pathways (Fig. 4B).
Taken together, these results indicate that hypermethylation of TSGs are associated with the activation of oncogenic pathways, chemoresistance and poor prognosis in HGSC patients.
Discussion
Chemotherapy treatment, despite being the first line of treatment for HGSC, has its limitation due to the resistance developed by HGSC patients. Major obstacle in the successful clinical management of HGSC chemotherapy is the lack of sensitive and specific biomarkers for the development of acquired drug resistance61. Emerging evidences has confirmed the indispensable role of DNA methylation in ovarian cancer chemoresistance18–21,62,63. Moreover, several pre-clinical studies have also indicated that demethylating agents treatment in solid tumor can reverse platinum chemoresistance including ovarian cancer64–67. Recently, pharmacological epigenetic has been center of attention for medical researchers especially DMPs and DMRs which are considered as worthwhile indicator to identify the molecular characteristics of cancer and chemoresistance development68. Therefore, in this study we attempted to analyze the methylation profile of multiple chemosensitive and chemoresistant HGSC cell lines by performing global methylation profiling to identify methylation differences associated with chemoresistance in HGSC. We used MethylationEPIC BeadChip microarray (HM850K; Illumina28 that can quantitatively analyze over 850,000 CpG position with single nucleotide resolution difference. We identified total of 3,641 DMPs between HGSC chemoresistant and chemosensitive cells, which included 2,939 (89%) hypermethylated DMPs in chemoresistant cells indicating that higher DNA methylation is associated with chemoresistance in HGSC. Our analysis also identified multiple key tumor suppressor genes (SOX17, ATP1A1, CD58, IFNGR1, etc.) and oncogenes (SF3B2, BCL2, SOS1, FURIN etc.) that have previously been identified as important in contributing to tumorigenesis and chemoresistance69–75.
Enrichment analysis indicated downregulation of multiple key genes associated with oncogenic pathways such as PIP5K1B and INPP4B of phosphatidylinositol signaling, BRCA1 of homologous recombination, LAMA3, TNR and SV2C of ECM-receptor interaction pathways. Phosphatidylinositol (PI3K/AKT) pathway has been showed to drive metastasis and drug resistance in multiple cancers where overactivation of PI3K/AKT pathway leads to upregulation of cell proliferation and cell migration76. BRCA1 expression is associated with multiple cellular process, including homologous recombination, DNA repair mechanism, chromatin remodelling and cell cycle regulation. The link between BRCA1 and chemotherapy resistance is context dependant. Promoter hypermethylation and low expression of BRCA1 was reported to enhance the platinum sensitivity in ovarian cancer cell lines and clinical studies77,78. However, in some studies BRCA1 methylation was associated with resistance to platinum drugs79,80. Our study identified hypomethylation and downregulation of BRCA1 in chemoresistant HGSC cell lines. Similarly, LAMA3 was also hypomethylated and downregulated in our study and have previously linked variably with platinum chemotherapy in multiple tumors including ovarian cancer. LAMA3 plays an important role in ECM-receptor interaction and regulate tumor mobility and invasiveness81. LAMA3 hypermethylation and low expression was linked with poor prognosis in ovarian cancer patients59, however in other study upregulation of LAMA3 was associated with low sensitivity to platinum therapy82. The BRCA1 and LAMA3 association with ovarian cancer chemoresistance needs further exploration. Our enrichment analysis also indicated downregulation of multiple other genes including WNT7A, WNT9A, IGF1, MSH2, GNAS, ALK that associated with cancer-related pathways. Consistently, these dysregulated pathways have been reported to promote cancer progression and chemoresistance development21,58–60.
Our study identified a specific set of TSGs, oncogenes and proto-oncogenes that were differentially methylated between HGSC resistant and sensitive cells. Moreover, when we looked for the correlation between differentially methylated genes and overall survival as well as progression-free survival of HGSC patients, we found that the differential methylation of CD58, SOX17, ETV1, FOXA1, and ATP1A was positively associated with poor prognosis in HGSC patients. These differentially methylated genes have been previously reported to be associated with cancer development, progression and chemoresistance and could be a potential DNA methylation signature for determining the HGSC chemoresistance. Our study identified significant CD58 hypermethylation in HGSC resistant cells compared to sensitive cells which is associated with decreased CD58 expression. CD58 is a highly glycosylated cell surface protein which is highly expressed in hematopoietic and non-hematopoietic cells83. CD58 plays a key role in increasing effector-target adhesion during antigen recognition. Effector-target adhesion is crucial in many immune responses including cytotoxicity, phagocytosis, and T-cell lymphocyte differentiation and proliferation84. Loss of CD58 expression can results in aberrant T-cell activation and diminish tumor killing effects85. Reduced CD58 expression could also cause immune evasion either by reduced effector-target adhesion or by upregulating PDL1 expression and results in resistance to immune checkpoint blockade therapy86 and CAR-T cells therapy87. Aberrant CD58 expression is implicated in tumor progression, and therapy response in multiple cancers including gastric carcinoma, hepatocellular carcinoma and lymphoma70,87,88. Further, SOX17 (SRY-box containing gene), a member of SOX transcription factor family, plays an important role in generation and maintenance of hematopoietic stem cells, canonical Wnt/β-catenin signaling pathways and in cancer development and metastasis89–91. SOX17 expression induced chemo-sensitization in multiple cancers including cholangiocarcinoma and esophageal cancer and is a promising biomarker in the cancer prognosis and treatment71,90,92. Low SOX17 expression in epithelial cancer cells was associated with decreased response to platinum treatment. SOX17 mediated the chemosensitivity through p53 regulated apoptosis by increasing the expression of caspase-9 and caspase-371. In other study, SOX17 affected concurrent chemoradiotherapy sensitivity in esophageal squamous cell carcinoma by transcriptional regulation of DNA repair and damage genes90. ETV1 is a member of oncogenic family of E26 transformation-specific (ETS) transcription factors that plays a key role in multiple physiological and pathological processes including embryogenesis, tissue reconstitution, and tumor progression93,94. ETV1 has been reported to function as an oncogene that regulates expression of genes that are associated with cell growth, migration, proliferation, differentiation and angiogenesis93. Numerous studies have shown that overactivation of ETV1 leads to cancer development and progression of various tumors including colorectal cancer, breast cancer, pancreatic cancer and gastrointestinal tumors95–98. Inhibiting ETV1 expressions in hepatocellular carcinoma enhanced the sensitivity to oxaliplatin treatment through miR-129-5p transcriptional regulation99. In another study, mutation in ETV1 conferred resistant to EGFR-tyrosine kinase inhibitors; standard treatment for non-small cell lung cancer100. Finally, FOXA1 (fork-head box A1) is a pioneer member of the FOX transcription factors family whose activation is important for proper development and differentiation of organs of endodermal origin including pancreas, lungs, liver, mammary glands and prostate101. FOXA1 also regulates glycolipid metabolism by controlling multiple genes expression in these organs102,103. Further studies have also demonstrated the role of FOXA1 in regulation of genes that are closely related to the cancer development and acquisition of chemoresistance in pancreatic and breast cancer104–107. We further explored the scope of our DNA methylation dataset by utilizing machine learning to investigate its association with overall survival and progression-free survival of HGSC patients. Consistently, hypermethylation in OVCA is significantly associated with chemoresistance and poor prognosis of HGSC patients which signifies the importance of DNA methylation in the outcome of chemotherapy response. We collected extensive information about methylation markers associated with ovarian cancer chemoresistance, however for future studies more comprehensive understanding could be achieved by integrating multi-omics approaches such as data from genomics, transcriptomics, proteomics and metabolomic.
Although our study provides important insight into DNA methylation association with HGSC chemoresistance, there are a few limitations that will be addressed in future studies. This study was conducted using small set of OVCA cell lines. Future studies will be extended to cover a larger panel of cell lines and clinical tumor samples. Our study identified multiple drug resistance driver genes, however functional and clinical significance of these genes in terms of ovarian cancer chemoresistance needs to be further explored. Many of the differentially methylated genes identified from our study are involved in important signalling pathways. It would be interesting to further explore how these signaling molecules may affect ovarian tumor progression and chemotherapy response. In addition, a key limitation of our machine learning approach is the relatively small training sample size. Although we applied robust dimensionality reduction (Recursive Feature Elimination) and regularization techniques (Elastic Net) to mitigate overfitting, the limited sample size inherently restricts model complexity, generalizability, and feature stability. Furthermore, the biological variability captured by a small panel of cell lines may not fully reflect the heterogeneity of clinical tumors. To strengthen such models’ predictive power and clinical applicability, future studies will require larger datasets with well-defined drug response annotations in patient samples for more rigorous and reliable validation. Nevertheless, the model’s ability to stratify patient outcomes and predict drug sensitivity in an independent cohort (TCGA-OV) supports its translational relevance. We also acknowledge that our study is limited in providing gene expression-based validation. Future studies leveraging datasets with matched expression and high-coverage methylation profiles, along with phenotype labels such as drug response, will be necessary to fully characterize the transcriptional consequences of chemoresistance-associated methylation signatures.
In summary, our global DNA methylation analysis revealed significant differences between chemosensitive and chemoresistant HGSC cells, with hypermethylation association with chemoresistance. CD58, SOX17. ETV1 and FOXA1 differential methylation was correlated with chemoresistance and poor patient outcomes. Notably, CD58 and ETV1 are two new identified genes linked to acquired chemoresistance in ovarian cancer. Our study expands the genetic marker pool for ovarian cancer chemoresistance and can be insightful for the development of novel epigenetic clinical biomarkers for HGSC chemotherapy resistance.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We acknowledge that Wide Methylation data was carried out at IARC in Dr. Zdenko Herceg’s lab under the funding Exploration NFRFE-(2019-01497) for ZH and CM. We thank Dr. Herceg and his colleagues for their tremendous support.
Author contributions
Conceptualization: H.B.M., M.A.-W., and B.K.T; Methodology: H.B.M, M.A.-W., and B.K.T.; Investigation and analysis: H.B.M., M.A., A.M., H.Y.-S., and C.M.; Resources, M.A.-W, A.M., C.M., and B.K.T.; Data curation: H.B.M., M.A., and A.M.; Writing—original draft preparation: H.B.M., and M.A.; Writing—review and editing: H.B.M., M.A., A.M., H.Y.-S., C.M., M.A.-W., and B.K.T.; Supervision: M.A.-W., and B.K.T.
Funding
This work was supported in part by the Canadian Institutes of Health Research (PJT-168949; awarded to BKT), Mitacs Globalink Research Award, Ovarian Cancer Canada (OCC) and Taggart-Parkes Fellowship (awarded to MA-W).
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hafiza Bushra Manzoor and Melisa Acun contributed equally to this work.
Contributor Information
Benjamin K. Tsang, Email: btsang@ohri.ca
Meshach Asare-Werehene, Email: mesh.asarewerehene@mail.utoronto.ca.
References
- 1.Sung, H. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.71(3), 209–249. 10.3322/caac.21660 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Allemani, C. et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet385(9972), 977–1010. 10.1016/S0140-6736(14)62038-9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torre, L. A. et al. Ovarian cancer statistics, 2018. CA Cancer J. Clin.68(4), 284–296. 10.3322/caac.21456 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Köbel, M. et al. An immunohistochemical algorithm for ovarian carcinoma typing. Int. J. Gynecol. Pathol.35(5), 430–441. 10.1097/PGP.0000000000000274 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffiths, C. T. & Fuller, A. F. Intensive surgical and chemotherapeutic management of advanced ovarian cancer. Surg. Clin. North Am.58(1), 131–142. 10.1016/S0039-6109(16)41440-4 (1978). [DOI] [PubMed] [Google Scholar]
- 6.Schwab, M. Encyclopedia of Cancer (Springer, 2008).
- 7.Agarwal, R. & Kaye, S. B. Ovarian cancer: strategies for overcoming resistance to chemotherapy. Nat. Rev. Cancer3(7), 502–516. 10.1038/nrc1123 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Gottesman, M. M. Mechanisms of cancer drug resistance. Annu. Rev. Med.53, 615–627. 10.1146/annurev.med.53.082901.103929 (2002). [DOI] [PubMed] [Google Scholar]
- 9.Pogge von Strandmann, E., Reinartz, S., Wager, U. & Müller, R. Tumor-host cell interactions in ovarian cancer: pathways to therapy failure. Trends Cancer3(2), 137–148. 10.1016/j.trecan.2016.12.005 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Beyes, S., Bediaga, N. G. & Zippo, A. An epigenetic perspective on intra-tumour heterogeneity: novel insights and new challenges from multiple fields. Cancers (Basel)13(19), 4969. 10.3390/cancers13194969 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gee, M. E., Faraahi, Z., McCormick, A. & Edmondson, R. J. DNA damage repair in ovarian cancer: unlocking the heterogeneity. J. Ovarian Res.11(1), 50. 10.1186/s13048-018-0424-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abedini, M. R. et al. Cell fate regulation by gelsolin in human gynecologic cancers. Proc. Natl. Acad. Sci. U.S.A.111(40), 14442–14447. 10.1073/pnas.1401166111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asare-Werehene, M. et al. The exosome-mediated autocrine and paracrine actions of plasma gelsolin in ovarian cancer chemoresistance. Oncogene39(7), 7. 10.1038/s41388-019-1087-9 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, P. A. & Baylin, S. B. The epigenomics of cancer. Cell128(4), 683–692. 10.1016/j.cell.2007.01.029 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma, S., Kelly, T. K. & Jones, P. A. Epigenetics in cancer. Carcinogenesis31(1), 27–36. 10.1093/carcin/bgp220 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones, P. A. DNA methylation and cancer. Oncogene21(35), 35. 10.1038/sj.onc.1205597 (2002). [DOI] [PubMed] [Google Scholar]
- 17.Baylin, S. B. & Ohm, J. E. Epigenetic gene silencing in cancer—a mechanism for early oncogenic pathway addiction?. Nat. Rev. Cancer6(2), 107–116. 10.1038/nrc1799 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Bonito, N. A., Borley, J., Wilhelm-Benartzi, C. S., Ghaem-Maghami, S. & Brown, R. Epigenetic regulation of the homeobox gene MSX1 associates with platinum resistant disease in high grade serous epithelial ovarian cancer. Clin. Cancer Res.22(12), 3097–3104. 10.1158/1078-0432.CCR-15-1669 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lund, R. J. et al. DNA methylation and transcriptome changes associated with cisplatin resistance in ovarian cancer. Sci. Rep.7, 1469. 10.1038/s41598-017-01624-4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chan, D. W. et al. Genome-wide DNA methylome analysis identifies methylation signatures associated with survival and drug resistance of ovarian cancers. Clin. Epigenet.13, 142. 10.1186/s13148-021-01130-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian, H. et al. Hypermethylation of mismatch repair gene hMSH2 associates with platinum-resistant disease in epithelial ovarian cancer. Clin. Epigenet.11, 153. 10.1186/s13148-019-0748-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaclavikova, R. et al. Development of high-resolution melting analysis for ABCB1 promoter methylation: Clinical consequences in breast and ovarian carcinoma. Oncol. Rep.42(2), 763. 10.3892/or.2019.7186 (2019). [DOI] [PubMed] [Google Scholar]
- 23.Mase, S. et al. ZNF671 DNA methylation as a molecular predictor for the early recurrence of serous ovarian cancer. Cancer Sci.110(3), 1105–1116. 10.1111/cas.13936 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan, Y., Liu, G., Zhou, F., Su, B. & Li, Y. DNA methylation profiles in cancer diagnosis and therapeutics. Clin. Exp. Med.18(1), 1–14. 10.1007/s10238-017-0467-0 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Létourneau, I. J. et al. Derivation and characterization of matched cell lines from primary and recurrent serous ovarian cancer. BMC Cancer12(1), 379. 10.1186/1471-2407-12-379 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viscarra, T. et al. Functional and transcriptomic characterization of carboplatin-resistant A2780 ovarian cancer cell line. Biol. Res.52(1), 13. 10.1186/s40659-019-0220-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wojtowicz, K. & Nowicki, M. The characterization of the sensitive ovarian cancer cell lines A2780 and W1 in response to ovarian CAFs. Biochem. Biophys. Res. Commun.662, 1–7. 10.1016/j.bbrc.2023.04.059 (2023). [DOI] [PubMed] [Google Scholar]
- 28.Moran, S., Arribas, C. & Esteller, M. Validation of a DNA methylation microarray for 850,000 CpG sites of the human genome enriched in enhancer sequences. Epigenomics8(3), 389–399. 10.2217/epi.15.114 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bošković, M. et al. DNA methylome changes of muscle- and neuronal-related processes precede bladder cancer invasiveness. Cancers (Basel)14(3), 487. 10.3390/cancers14030487 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vicente, A. L. S. A. et al. Cutaneous and acral melanoma cross-OMICs reveals prognostic cancer drivers associated with pathobiology and ultraviolet exposure. Nat. Commun.13(1), 4115. 10.1038/s41467-022-31488-w (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aryee, M. J. et al. Minfi: a flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics30(10), 1363–1369. 10.1093/bioinformatics/btu049 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen, M. maxprobes 2024. https://github.com/markgene/maxprobes (2024).
- 33.Ritchie, M. E. et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res.43(7), e47. 10.1093/nar/gkv007 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilhelm-Benartzi, C. S. et al. Review of processing and analysis methods for DNA methylation array data. Br. J. Cancer109(6), 1394–1402. 10.1038/bjc.2013.496 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peters, T. J. et al. De novo identification of differentially methylated regions in the human genome. Epigenetics Chromatin8, 6. 10.1186/1756-8935-8-6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cavalcante, R. G. & Sartor, M. A. annotatr: genomic regions in context. Bioinformatics33(15), 2381–2383. 10.1093/bioinformatics/btx183 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.IlluminaHumanMethylationEPICanno.ilm10b4.hg19. Bioconductor. http://bioconductor.org/packages/IlluminaHumanMethylationEPICanno.ilm10b4.hg19/ (2024).
- 38.The Cancer Genome Atlas Program (TCGA)—NCI. https://www.cancer.gov/ccg/research/genome-sequencing/tcga (2025).
- 39.Bibikova, M. et al. Genome-wide DNA methylation profiling using Infinium® assay. Epigenomics1(1), 177–200. 10.2217/epi.09.14 (2009). [DOI] [PubMed] [Google Scholar]
- 40.OncoKB™ Cancer Gene List. https://www.oncokb.org/cancer-genes (2024).
- 41.Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics32(18), 2847–2849. 10.1093/bioinformatics/btw313 (2016). [DOI] [PubMed] [Google Scholar]
- 42.EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Colouring and Labeling. https://bioconductor.org/packages/devel/bioc/vignettes/EnhancedVolcano/inst/doc/EnhancedVolcano.html (2024).
- 43.Gu, Z., Gu, L., Eils, R., Schlesner, M. & Brors, B. circlize Implements and enhances circular visualization in R. Bioinformatics30(19), 2811–2812. 10.1093/bioinformatics/btu393 (2014). [DOI] [PubMed] [Google Scholar]
- 44.OncoKB™—MSK’s Precision Oncology Knowledge Base. OncoKB™. https://www.oncokb.org/ (2024).
- 45.Valero-Mora, P. M. ggplot2: Elegant graphics for data analysis. J. Stat. Softw.35, 1–3. 10.18637/jss.v035.b01 (2010).21603108 [Google Scholar]
- 46.Yu, G. Chap. 15 Visualization of Functional Enrichment Result|Biomedical Knowledge Mining Using GOSemSim and clusterProfiler. https://yulab-smu.top/biomedical-knowledge-mining-book/enrichplot.html (2024).
- 47.Drawing Survival Curves using ggplot2—survminer. https://rpkgs.datanovia.com/survminer/ (2024).
- 48.Ulgen, E., Ozisik, O. & Sezerman, O. U. pathfindR: An R package for comprehensive identification of enriched pathways in omics data through active subnetworks. Front. Genet.10, 858. 10.3389/fgene.2019.00858 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanehisa, M. & Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res.28(1), 27–30. 10.1093/nar/28.1.27 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.The Gene Ontology Consortium, “The Gene Ontology knowledgebase in 2023,” Genetics, vol. 224, no. 1, p. iyad031, 2023, doi: 10.1093/genetics/iyad031. [DOI] [PMC free article] [PubMed]
- 51.Ashburner, M. et al. Gene Ontology: tool for the unification of biology. Nat. Genet.25(1), 25–29. 10.1038/75556 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.T. Wu et al., “clusterProfiler 4.0: A universal enrichment tool for interpreting omics data,” Innovation (Camb), vol. 2, no. 3, p. 100141, 2021, doi: 10.1016/j.xinn.2021.100141. [DOI] [PMC free article] [PubMed]
- 53.Kuhn, M. Building predictive models in R using the caret package. J. Stat. Softw.28, 1–26. 10.18637/jss.v028.i05 (2008).27774042 [Google Scholar]
- 54.Gene Selection for Cancer Classification Using Support Vector Machines|Machine Learning. https://link.springer.com/article/10.1023/A:1012487302797 (2024).
- 55.Friedman, J., Hastie, T. & Tibshirani, R. Regularization paths for generalized linear models via coordinate descent. J. Stat. Softw.33(1), 1–22 (2010). [PMC free article] [PubMed] [Google Scholar]
- 56.Dudley, W. N., Wickham, R. & Coombs, N. An introduction to survival statistics: Kaplan-Meier analysis. J. Adv. Pract. Oncol.7(1), 91–100 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ando, M. et al. Chromatin dysregulation and DNA methylation at transcription start sites associated with transcriptional repression in cancers. Nat. Commun.10, 2188. 10.1038/s41467-019-09937-w (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jacot, W. et al. BRCA1 promoter hypermethylation is associated with good prognosis and chemosensitivity in triple-negative breast cancer. Cancers (Basel)12(4), 828. 10.3390/cancers12040828 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feng, L., Huang, Y., Zhang, W. & Li, L. LAMA3 DNA methylation and transcriptome changes associated with chemotherapy resistance in ovarian cancer. J. Ovarian Res.14, 67. 10.1186/s13048-021-00807-y (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hao, J. et al. TCL1A acts as a tumour suppressor by modulating gastric cancer autophagy via miR-181a-5p-TCL1A-Akt/mTOR-c-MYC loop. Carcinogenesis44(1), 29–37. 10.1093/carcin/bgac085 (2023). [DOI] [PubMed] [Google Scholar]
- 61.Chandra, A. et al. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med.8(16), 7018–7031. 10.1002/cam4.2560 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lum, E. et al. Loss of DOK2 induces carboplatin resistance in ovarian cancer via suppression of apoptosis. Gynecol. Oncol.130(2), 369–376. 10.1016/j.ygyno.2013.05.002 (2013). [DOI] [PubMed] [Google Scholar]
- 63.H. Cardenas et al., “Methylomic signatures of high grade serous ovarian cancer,” Epigenetics, vol. 16, no. 11, pp. 1201–1216, doi: 10.1080/15592294.2020.1853402. [DOI] [PMC free article] [PubMed]
- 64.Fang, F. et al. A phase 1 and pharmacodynamic study of decitabine in combination with carboplatin in patients with recurrent, platinum-resistant, epithelial ovarian cancer. Cancer116(17), 4043–4053. 10.1002/cncr.25204 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu, S. et al. Phase 1b–2a study to reverse platinum resistance through use of a hypomethylating agent, azacitidine, in patients with platinum-resistant or platinum-refractory epithelial ovarian cancer. Cancer117(8), 1661–1669. 10.1002/cncr.25701 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu, J. F. et al. Safety, clinical activity and biomarker assessments of atezolizumab from a Phase I study in advanced/recurrent ovarian and uterine cancers. Gynecol. Oncol.154(2), 314–322. 10.1016/j.ygyno.2019.05.021 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Oza, A. M. et al. A randomized phase II trial of epigenetic priming with guadecitabine and carboplatin in platinum-resistant, recurrent ovarian cancer. Clin. Cancer Res.26(5), 1009–1016. 10.1158/1078-0432.CCR-19-1638 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Choudhury, S. R. et al. The functional epigenetic landscape of aberrant gene expression in molecular subgroups of newly diagnosed multiple myeloma. J. Hematol. Oncol.13(1), 108. 10.1186/s13045-020-00933-y (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Huang, W. et al. Comprehensive analysis of the expression of sodium/potassium-ATPase α subunits and prognosis of ovarian serous cystadenocarcinoma. Cancer Cell Int.20, 309. 10.1186/s12935-020-01414-5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yan, X. et al. CD58 loss in tumor cells confers functional impairment of CAR T cells. Blood Adv.6(22), 5844–5856. 10.1182/bloodadvances.2022007891 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang, Y. et al. SOX17 increases the cisplatin sensitivity of an endometrial cancer cell line. Cancer Cell Int.16, 29. 10.1186/s12935-016-0304-7 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tecalco-Cruz, A. C. et al. Deregulation of interferon-gamma receptor 1 expression and its implications for lung adenocarcinoma progression. World J. Clin. Oncol.15(2), 195–207. 10.5306/wjco.v15.i2.195 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Daley, B. R. et al. SOS1 inhibition enhances the efficacy of and delays resistance to G12C inhibitors in lung adenocarcinoma. BioRxiv7, 570642. 10.1101/2023.12.07.570642 (2023). [Google Scholar]
- 74.Maji, S. et al. Bcl-2 antiapoptotic family proteins and chemoresistance in cancer. Adv. Cancer Res.137, 37–75. 10.1016/bs.acr.2017.11.001 (2018). [DOI] [PubMed] [Google Scholar]
- 75.Xu, H. et al. RING-finger protein 6 promotes colorectal tumorigenesis by transcriptionally activating SF3B2. Oncogene40(47), 6513–6526. 10.1038/s41388-021-01872-9 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maloney, S. M., Hoover, C. A., Morejon-Lasso, L. V. & Prosperi, J. R. Mechanisms of taxane resistance. Cancers (Basel)12(11), 3323. 10.3390/cancers12113323 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stefansson, O. A., Villanueva, A., Vidal, A., Martí, L. & Esteller, M. BRCA1 epigenetic inactivation predicts sensitivity to platinum-based chemotherapy in breast and ovarian cancer. Epigenetics7(11), 1225–1229. 10.4161/epi.22561 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ignatov, T. et al. BRCA1 promoter methylation is a marker of better response to platinum-taxane-based therapy in sporadic epithelial ovarian cancer. J. Cancer Res. Clin. Oncol.140(9), 1457–1463. 10.1007/s00432-014-1704-5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Patch, A.-M. et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature521(7553), 489–494. 10.1038/nature14410 (2015). [DOI] [PubMed] [Google Scholar]
- 80.Wang, Y.-Q. et al. Epigenetic inactivation of BRCA1 through promoter hypermethylation in ovarian cancer progression. J. Obstet. Gynaecol. Res.39(2), 549–554. 10.1111/j.1447-0756.2012.01979.x (2013). [DOI] [PubMed] [Google Scholar]
- 81.Peng, X., Yu, M. & Chen, J. Transcriptome sequencing identifies genes associated with invasion of ovarian cancer. J. Int. Med. Res.48(9), 0300060520950912. 10.1177/0300060520950912 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li, J., Yu, Y., Zeng, R., Zou, Y. & Bu, J. Knockdown of LAMA3 enhances the sensitivity of colon cancer to oxaliplatin by regulating the Hippo-YAP pathway. Biochem. Biophys. Acta.1871(3), 167665. 10.1016/j.bbadis.2025.167665 (2025). [DOI] [PubMed] [Google Scholar]
- 83.Möller, P., Koretz, K., Schlag, P. & Momburg, F. Frequency of abnormal expression of HLA-A,B, C and HLA-DR molecules, invariant chain, and LFA-3 (CD58) in colorectal carcinoma and its impact on tumor recurrence. Int. J. Cancer Suppl.6, 155–162. 10.1002/ijc.2910470727 (1991). [DOI] [PubMed] [Google Scholar]
- 84.Moingeon, P. et al. CD2-mediated adhesion facilitates T lymphocyte antigen recognition function. Nature339(6222), 312–314. 10.1038/339312a0 (1989). [DOI] [PubMed] [Google Scholar]
- 85.Frangieh, C. J. et al. Multi-modal pooled Perturb-CITE-Seq screens in patient models define novel mechanisms of cancer immune evasion. Nat. Genet.53(3), 332–341. 10.1038/s41588-021-00779-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jerby-Arnon, L. et al. A cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell175(4), 984–997. 10.1016/j.cell.2018.09.006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Younes, S. et al. Detection of aberrant CD58 expression in a wide spectrum of lymphoma subtypes: implications for treatment resistance. Mod. Pathol.36(10), 100256. 10.1016/j.modpat.2023.100256 (2023). [DOI] [PubMed] [Google Scholar]
- 88.Wang, C. et al. CD58 acts as a tumor promotor in hepatocellular carcinoma via activating the AKT/GSK-3β/β-catenin pathway. J. Transl. Med.21(1), 539. 10.1186/s12967-023-04364-4 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Engert, S. et al. Wnt/β-catenin signalling regulates Sox17 expression and is essential for organizer and endoderm formation in the mouse. Development140(15), 3128–3138. 10.1242/dev.088765 (2013). [DOI] [PubMed] [Google Scholar]
- 90.Kuo, I.-Y. et al. SOX17 overexpression sensitizes chemoradiation response in esophageal cancer by transcriptional down-regulation of DNA repair and damage response genes. J. Biomed. Sci.26(1), 20. 10.1186/s12929-019-0510-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim, I., Saunders, T. L. & Morrison, S. J. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell130(3), 470–483. 10.1016/j.cell.2007.06.011 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lozano, E. et al. MRP3-mediated chemoresistance in cholangiocarcinoma: target for chemosensitization through restoring SOX17 expression. Hepatology72(3), 949–964. 10.1002/hep.31088 (2020). [DOI] [PubMed] [Google Scholar]
- 93.Sizemore, G. M., Pitarresi, J. R., Balakrishnan, S. & Ostrowski, M. C. The ETS family of oncogenic transcription factors in solid tumours. Nat. Rev. Cancer17(6), 337–351. 10.1038/nrc.2017.20 (2017). [DOI] [PubMed] [Google Scholar]
- 94.Peng, Y. et al. The role of E26 transformation-specific variant transcription factor 5 in colorectal cancer cell proliferation and cell cycle progression. Cell Death Dis.12(5), 427. 10.1038/s41419-021-03717-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li, J. et al. miR-17-5p suppresses cell proliferation and invasion by targeting ETV1 in triple-negative breast cancer. BMC Cancer17(1), 745. 10.1186/s12885-017-3674-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heeg, S. et al. ETS-Transcription Factor ETV1 Regulates Stromal Expansion and Metastasis in Pancreatic Cancer. Gastroenterology151(3), 540–553. 10.1053/j.gastro.2016.06.005 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oh, S., Song, H., Freeman, W. M., Shin, S. & Janknecht, R. Cooperation between ETS transcription factor ETV1 and histone demethylase JMJD1A in colorectal cancer. Int. J. Oncol.57(6), 1319–1332. 10.3892/ijo.2020.5133 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guo, X. et al. ETV1 inhibition depressed M2 polarization of tumor-associated macrophage and cell process in gastrointestinal stromal tumor via down-regulating PDE3A. J. Clin. Biochem. Nutr.72(2), 139–146. 10.3164/jcbn.22-47 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen, J., Yuan, D., Hao, Q., Zhu, D. & Chen, Z. LncRNA PCGEM1 mediates oxaliplatin resistance in hepatocellular carcinoma via miR-129-5p/ETV1 axis in vitro. Advances in Clinical and Experimental Medicine30, 8. 10.17219/acem/135533 (2021). [DOI] [PubMed] [Google Scholar]
- 100.Zhou, Y. et al. Novel ETV1 mutation in small cell lung cancer transformation resistant to EGFR tyrosine kinase inhibitors. Ann. Transl. Med.9(14), 1150. 10.21037/atm-21-2625 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Geusz, R. J. et al. Sequence logic at enhancers governs a dual mechanism of endodermal organ fate induction by FOXA pioneer factors. Nat. Commun.12(1), 6636. 10.1038/s41467-021-26950-0 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Juan-Mateu, J. et al. Pancreatic microexons regulate islet function and glucose homeostasis. Nat. Metab.5(2), 219–236. 10.1038/s42255-022-00734-2 (2023). [DOI] [PubMed] [Google Scholar]
- 103.Kaestner, K. H. The hepatocyte nuclear factor 3 (HNF3 or FOXA) family in metabolism. Trends Endocrinol. Metab.11(7), 281–285. 10.1016/s1043-2760(00)00271-x (2000). [DOI] [PubMed] [Google Scholar]
- 104.Chen, X., Li, M., Zhou, H. & Zhang, L. miR-132 targets FOXA1 and exerts tumor-suppressing functions in thyroid cancer. Oncol. Res.27(4), 431–437. 10.3727/096504018X15201058168730 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.He, S., Zhang, J., Zhang, W., Chen, F. & Luo, R. FOXA1 inhibits hepatocellular carcinoma progression by suppressing PIK3R1 expression in male patients. J. Exp. Clin. Cancer Res.36(1), 175. 10.1186/s13046-017-0646-6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kumar, U., Ardasheva, A., Mahmud, Z., Coombes, R. C. & Yagüe, E. FOXA1 is a determinant of drug resistance in breast cancer cells. Breast Cancer Res. Treat.186(2), 317–326. 10.1007/s10549-020-06068-5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang, Y. et al. A novel lncRNA MCM3AP-AS1 promotes the growth of hepatocellular carcinoma by targeting miR-194-5p/FOXA1 axis. Mol. Cancer18(1), 28. 10.1186/s12943-019-0957-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.