Abstract
Background
Safe and effective vaccines are a key preventative measure to protect infants from SARS-CoV-2 infection and disease. Although mRNA vaccines induce robust antibody titers in infants, little is known about the quality of CD4 T-cell responses induced by vaccination. CD4 T-cell responses are important in orchestrating coordinated immune responses during infection and may help to limit disease severity.
Methods
To characterize the CD4 T-cell response to SARS-CoV-2 mRNA vaccination in infants, we sampled blood from 13 infants before and after primary SARS-CoV-2 mRNA vaccine series; samples from 12 historical vaccinated adults were used for comparisons. Peripheral blood mononuclear cells were stimulated with spike peptide pools, and the ability of CD4 T-cells to secrete Th1, Th2, and Th17 cytokines was quantified. Measures of polyfunctionality were generated with the COMPASS algorithm.
Results
We observed a significant increase in CD4 T-cells producing IL-2 (0.01% vs 0.08%, P = .04) and TNF-α (0.007% vs 0.07%, P = .007) following vaccination in infants but a more muted induction of IFN-γ production (0.01% vs 0.04%, P = .08). This contrasted with adults, in whom vaccination induced robust production of IFN-γ, IL-2, and TNF-α. Th2 and Th17 responses were limited in both infants and adults. In infants, CD4 T-cell responses post vaccination were greater in those who received mRNA-1273 vs BNT162b. In contrast to CD4 T-cell responses, spike-specific IgG titers were similar in infants and adults.
Conclusions
These data suggest that infants have restricted induction of cytokine-producing CD4 T-cells following SARS-CoV-2 mRNA vaccination relative to adults.
Keywords: cellular immunity, infants, pediatric immunity, SARS-CoV-2, vaccination
Vaccination against SARS-CoV-2 is a critical intervention for the prevention of morbidity and mortality associated with COVID-19 disease. The mRNA-based vaccines BNT162b and mRNA-1273 were initially developed for adults but subsequently adapted with an altered mRNA amount and vaccine schedule for pediatric populations >6 months of age [1]. Immunogenicity comparison studies indicated that mRNA vaccines induced comparable anti-spike IgG titers in infants and adults [2, 3], but efficacy against symptomatic COVID-19 was only 75.8% in children aged 6 months to 2 years for BNT162b and 50.6% for mRNA-1273 [2, 3], lower than early studies in adults [4, 5].
Generation of spike-specific antibodies, particularly those that neutralize virus, has been the predominant focus of vaccine efforts [3, 6, 7], as antibodies are critical to preventing infection [8]. However, upon infection, viral clearance and control of disease may be primarily T- cell mediated [9–14]. T-cell responses may also be less susceptible to immune escape relative to antibodies [13, 15]. In adults, studies have identified CD4 and CD8 T-cell responses following SARS-CoV-2 mRNA vaccination, although CD4 responses are predominant [15–18]. In contrast, few prior studies have investigated T-cell responses generated by SARS-CoV-2 mRNA vaccines in infants [19]. This may reflect the challenge of obtaining sufficient whole blood from infants to conduct T-cell stimulation assays, compounded by the low frequency of antigen-specific T-cells induced by vaccination [20, 21].
To address this critical knowledge gap, we characterized the infant CD4 T-cell response to primary SARS-CoV-2 mRNA vaccination, including frequency and functionality, using a group of adult vaccine recipients as reference.
MATERIAL AND METHODS
Patient Consent Statement and Cohort Details
Maternal-infant dyads were enrolled into the MATIMM study, which was approved by the University of Washington Institutional Review Board (STUDY00008491); for infant participation, informed consent was obtained from mothers. Families were identified who intended to vaccinate their infant at <12 months of age with a SARS-CoV-2 mRNA vaccine. The infant formulation of BNT162b is given as a 3-dose series and contains 3 µg of mRNA per dose, whereas mRNA-1273 is given as a 2-dose series and contains 25 µg of mRNA per dose [22, 23]. Peripheral blood was collected from infants before the first vaccine dose and again 3 to 7 weeks following completion of the primary series. Whole blood was processed for plasma and peripheral blood mononuclear cells (PBMC) as previously described [24]. Clinical data were abstracted from patient charts.
We utilized plasma and PBMC from adults vaccinated with the BNT162b or mRNA-1273 primary series, as banked by the HAARVI Study (UW: STUDY00000959) [25], SCRI SARS2 Vaccine Study (SC: STUDY00003064), or the SCRI CGIDR Biorepository (SC: STUDY00002048) [26]. For all adult samples, informed consent was obtained by the parent study, and allowance for subsequent use of the banked samples in future studies was included within the protocol. Samples were collected before vaccination and 2 to 7 weeks following completion of the primary series. Adults were selected to include an equal distribution of individuals vaccinated with BNT162b or mRNA-1273. The primary series at the time of sample collection consisted of a 2-dose series for each vaccine. BNT162b contains 30 µg of mRNA per dose, whereas mRNA-1273 contains 100 µg of mRNA per dose [22, 23].
Plasma for all adult samples and all but 1 infant pre-vaccine sample was tested for SARS-CoV-2 nucleocapsid (NC)–IgG by enzyme-linked immunosorbent assay. For the single infant exception, plasma had previously tested negative for NC-IgG and positive for spike-IgG via Elecsys Anti-SARS-CoV-2 (Roche Diagnostics GmbH).
Intracellular Cytokine Staining
PBMC were thawed at 37 °C and transferred to prewarmed RPMI 1640 with L-glutamine (Thermo Fisher) with 10% fetal bovine serum (Biowest) and 0.2% Benzonase (Millipore Sigma). Cells were rested overnight at 37 °C with 5% CO2. The next day, cells were enumerated, resuspended in medium, and split across 3 wells of a 96-well U-bottom plate at a density of 1 to 2 million cells per well. S1 and S2 peptide pools (pp) optimized for CD4 T-cell stimulation containing 15mers with 11aa overlap (JPT Peptide Technologies) were prepared [27]. Stimulation cocktails were prepared for the conditions of negative control: 0.2% DMSO (Sigma-Aldrich), experimental condition: spike pp (1 μg/mL of each S1 and S2), and positive control: 0.05-μg/mL phorbol-12-myristate-13-acetate and 0.7471-μg/mL ionomycin. Stimulation cocktails also contained 10-μg/mL brefeldin A (Sigma-Aldrich), GolgiStop (BD Biosciences), 1-μg/mL CD28/49d (BD Biosciences), and anti-CD107a PE-Cy7 (BD Biosciences). Cells were stimulated for 6 hours at 37 °C, followed by the addition of EDTA, and overnight storage at 4 °C [20 ]. Cells were subsequently stained with Fixable Aqua (Invitrogen) and anti-CCR7 (BD Biosciences), followed by red blood cell lysis with FACS Lyse (BD Biosciences). Cells were permeabilized with FACS Perm II (BD Biosciences) and stained with an antibody cocktail (Supplementary Table 1), fixed with 1% paraformaldehyde, and then resuspended in phosphate-buffered saline (PBS) with 2mM EDTA. The antibody panel included extracellular lineage (CD3, CD14, CD19), subset (CD4, CD8, CD45RA), and activation (CD38, CD154, HLADR) markers and intracellular cytokines (IFNγ, TNFα, IL-2, IL-4, IL-5, IL-13, and IL-17). Data were acquired on a BD LSR Fortessa flow cytometer. Adult positive and negative biological controls were run in each experiment, and the pre- and post-vaccine time point samples from each individual were run together.
Flow Cytometry Data Analysis
Flow cytometry data were analyzed by FlowJo software (version 10.10). The gating strategy is presented in Supplementary Figure 1. Briefly, the live, single, CD3+/CD4+/CD19–/CD14– population was identified. To compare monofunctional cytokine production, the background frequency of cells producing a cytokine in the DMSO condition was subtracted from that of the spike pp condition. COMPASS (version 1.32.0; Combinatorial Polyfunctionality Analysis of Ag-Specific T-Cell Subsets) [28] was used to analyze the functional profiles of antigen-specific T-cells in an unbiased and comprehensive manner. COMPASS outputs a functionality score (FS), which estimates the proportion of antigen-specific cell subsets weighted by the degree of functionality, and a polyfunctionality score (PFS), which summarizes the breath of polyfunctional responses. Outputs generated by COMPASS (unique subsets, FS, PFS) have been used as a measure of CD4 T-cell responses to SARS2 [19, 27], HIV [28], bacille Calmette-Guérin [29], and novel tuberculosis vaccination [30] and are associated with protection from HIV [28] and tuberculosis [31]. Default settings were used to filter categories, and samples with <3000 CD4 T-cells were excluded from analysis. The R package ComplexHeatmap (version 2.10.0) was used to visualize the probabilities of response from the COMPASS output [32]. One pre-vaccine infant sample did not meet the cell count threshold and was excluded.
Enzyme-Linked Immunosorbent Assay
Plasma IgG titers to SARS-CoV-2 trimer and NC were determined through direct immobilization enzyme-linked immunosorbent assay. Plasma was heat inactivated for 1 hour at 56 °C before being centrifuged at 17 000g for 10 minutes. Immulon 2HB 96-well plates (3455; Thermo Scientific) were coated with 50 ng/well of SARS-CoV-2 trimer or NC in 0.1M aHCO3, pH 9.5, overnight at room temperature. Plates were washed between steps with PBS containing 0.2% Tween 20. Coated plates were blocked with PBS, 10% nonfat milk, and 0.3% Tween 20. Following blocking, plasma samples were serially diluted over a range of 1:50 to 1:36 450 in PBS, 10% nonfat milk, and 0.03% Tween 20 and incubated for 1 hour at 37 °C. Bound antibodies were detected by goat anti-human IgG Fc-HRP (2049-05; Southern Biotech) at 1:4000 dilution. Plates were developed by using 50 μL of TMB Peroxidase Substrate (5120-0083; SeraCare Life Sciences Inc) and then stopped after 3 minutes with 50 μL of 1N H2SO4. Absorbance at 450 nm was determined with a BioTek ELx800 microplate reader. Endpoint titers were defined as the reciprocal of plasma dilution at an optical density of 0.1 after the subtraction of plate background. A negative serologic response was defined as <1:50.
Statistical Analysis
The magnitude of background-subtracted CD4 T-cell cytokine responses was compared between pre- and post-vaccine samples with paired t tests. For comparison of post-vaccine response (individual cytokines, FS, PFS, and spike-specific IgG) between infants and adults a multivariate regression model was utilized with adjustment for sex, time postvaccination, and vaccine manufacturer. For comparison of post-vaccine response by vaccine manufacturer a multivariate model was utilized separately for infants or adults with adjustment for sex, time postvaccination, and age at series initiation. The infant who received a mixed vaccine series was excluded from this analysis. For comparison of endpoint titers, a value of 1:49 was used for samples negative at the initial 1:50 dilution. CD4 T-cell FS vs spike endpoint titer was compared with Pearson correlation.
RESULTS
Cohort Characteristics
We enrolled 13 infants who received a primary series of either SARS-CoV-2 mRNA vaccine, which included 1 set of twins. There were 4 female and 9 male infants. The median age of infants at the pre-vaccine time point was 6.9 months (range, 6–11.5; Table 1), which corresponded to a median of 1 day (range, 0–16) prior to receipt of first vaccine dose. Six infants received the 2-dose mRNA-1273 vaccine, 6 received the 3-dose BNT162b vaccine, and 1 infant received 2 doses of BNT162b followed by 1 dose of mRNA-1273. Infants were a median of 10.1 months old (range, 7.9–15.1) at the post-vaccine time point, which corresponded to a median of 28 days (range, 22–42) after completion of the primary vaccine series (Figure 1).
Table 1.
Participant Characteristics
| No. (%) or Median (Range) | ||
|---|---|---|
| Infants (n = 13) | Adults (n = 12) | |
| Sex | ||
| Female | 4 (31) | 7 (58) |
| Male | 9 (69) | 5 (42) |
| Age at vaccine series initiation | 6.9 mo (6.0–11.5) | 37.5 y (27–81) |
| Vaccine type | ||
| BNT162b | 6 (46) | 6 (50) |
| mRNA-1273 | 6 (46) | 6 (50) |
| Mixed | 1 (8) | … |
| Time between events (days) | ||
| Pre-vaccine blood draw and first vaccine dose | 1 (0–16) | 52 (2–323) |
| Final vaccine dose and post-vaccine blood draw | 28 (22–42) | 14.5 (9–42) |
Figure 1.
Vaccination overview. Infants (n = 13) and adults (n = 12) donated blood prior to and following SARS-CoV-2 vaccination. BNT162b2 primary series for infants included 3 doses; mRNA-1273 primary series for infants included 2 doses. One infant received 2 doses of BNT162b vaccine and 1 dose of the mRNA-1273 vaccine (not represented). Both adult primary series included 2 doses. Time points are relative to the first vaccine dose and represent medians. Created with BioRender.com.
The median age of adult controls was 37.5 years (range, 27–81 years; Table 1); 7 were female and 5 were male. Six individuals received BNT162b and 6 received mRNA-1273. The pre-vaccine blood draw occurred at a median 52 days (range, 2–323) prior to the first dose of vaccine (Figure 1). The median time of blood draw following completion of the primary series was 14.5 days (range, 9–42). There was no significant difference in age or sex by vaccine type for either infants or adults.
CD4 T-Cell Cytokine Responses to SARS-CoV-2 Spike Following mRNA Vaccination
Our primary aim was to characterize the infant CD4 T-cell response to SARS-CoV-2 mRNA vaccines. In the 13 infants sampled, there were universally low CD4 T-cell responses to spike stimulation at the pre-vaccine time point. Between the pre- and post-vaccine time points, there were significant increases in the frequency of CD4 T-cells producing IL-2 (0.01% vs 0.08%, P = .04) and TNF-α (0.007% vs 0.07%, P = .007) in response to spike pp but a more muted induction of IFNγ (0.01% vs 0.04%, P = .08; Figure 2, Supplementary Table 2). In addition, there were low frequencies of CD4 T-cells producing Th2 cytokines (0.005% vs 0.03%, P = .07) or IL-17a (0.008% vs 0.02%, P = .2) in response to spike stimulation, but these responses were not significantly different pre- and post-vaccine. CD4 T-cells also significantly increased their expression of CD154 following stimulation with spike pp between pre- and post-vaccine time points (0.04% vs 0.15%, P = .04).
Figure 2.
Cytokine production by CD4 T -ells before and after SARS-CoV-2 mRNA vaccination. A, Representative flow plots of infant CD4 T -ells expressing Th1, Th2, and Th17 cytokines or CD154 in response to stimulation with DMSO or spike peptide pool. B, Summary plots of background-subtracted frequencies of infant CD4 T-cells expressing cytokines in response to stimulation with spike peptide pool. Background-subtracted cytokine responses pre- vs post-vaccination were compared with paired t tests. Post-vaccine responses between adults and infants were compared with a multivariate regression model with adjustment for sex, time post-accination, and vaccine manufacturer. *P ≤ .05. **P ≤ .01. ns, not significant.
In adults we observed a robust increase in CD4 T-cell production of all Th1 cytokines in response to spike pp between the pre- and post-vaccine time points (IFNγ: 0.02% vs 0.26%, P = .03; IL-2: 0.004% vs 0.44%, P = .05; TNF-α: 0.002% vs 0.40%, P = .08), although TNF-α was not statistically significant, consistent with prior reports [20]. In addition, there was an increase in the frequency of CD4 T-cells producing Th2 cytokines (0.006% vs 0.06%, P = .02) but not IL-17a (0% vs 0.02%, P = .2). There was a nonsignificant increase in the frequency of CD4 T-cells expressing CD154 (0.03% vs 0.87%, P = .09).
At the post-vaccine time point, IFNγ production was significantly higher among adults vs infants (adjusted difference, 0.26%; P = .04). Production of TNF-α (adjusted difference, 0.40%; P = .1) and IL-2 (0.40%, P = .1) and expression of CD154 (0.86%, P = .1) were also higher but did not meet significance (Figure 2B).
CD4 T-Cell Polyfunctionality Following SARS-CoV-2 mRNA Vaccination Varies Between Infants and Adults
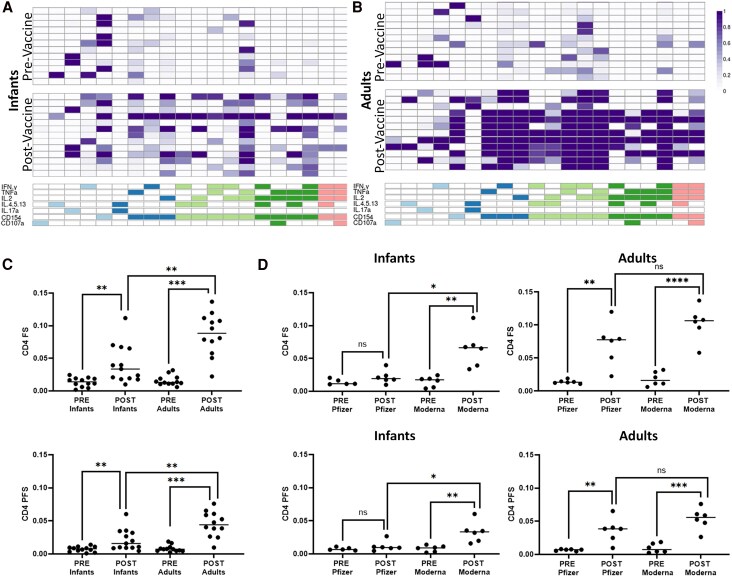
To comprehensively assess the functional diversity of SARS-CoV-2 vaccine-induced CD4 T-cell responses among infants, we used COMPASS to determine the probability of detecting a response greater than the background among all possible combinations of cytokine subsets. A response was detected in 20 of 107 possible CD4 functional subsets, 15 of which were polyfunctional (Figure 3A and 3B). Several spike-specific CD4 T-cell subsets were identified at the pre-vaccine timepoint, which may be due to cross-reactivity between SARS-CoV-2 spike protein and other common human coronaviruses [33 ]. Post-vaccination, a polyfunctional subset producing canonical Th1 cytokines IFN-γ, IL-2, and TNF-α, in addition to CD154, was identified in 10 of 13 infant samples with moderate probability scores and 11 of 12 adult samples with high probability scores. Similarly, a polyfunctional subset positive for IL-2, TNF-α, and CD154 was identified in all infant samples and 11 of 12 adult samples, and a subset with IFN-γ, IL-2, and CD154 was present in 5 infants with mostly low probability scores and all adult samples with high probability scores (Figure 3B). IL-17a was present in only 2 subsets: a monofunctional subset and a polyfunctional subset in combination with Th2 cytokines. Together, these data underscore the Th1 skew of CD4 T-cells in response to SARS-CoV-2 vaccination in both infants and adults and the robust production of IFNγ in adults.
Figure 3.
Functional breath of CD4 T-cell response determined by COMPASS analysis. A, Results from COMPASS analysis of ICS data from infants are displayed as a heat map of probabilities. Rows represent individuals, with order matched between pre- and post-vaccine groups. Columns represent functional CD4 T-cell subsets identified in an unbiased manner by COMPASS. Columns of the same color share the same degree of functionality. B, Probability heat map output from COMPASS analysis of ICS data from adults. C, FS and PFS by age group and vaccine status. D, FS and PFS arranged by vaccine status and vaccine manufacturer for infants and adults. Post-vaccine response by vaccine manufacturer was compared with multivariate regression with adjustment for sex, time post-vaccination, and age at vaccine series initiation, separately for adults or infants. *P < .05. **P < .01. ***P < .001. ns, not significant. COMPASS, Combinatorial Polyfunctionality Analysis of Ag-Specific T-cell Subsets; FS, functionality score; ICS, intracellular cytokine staining; PFS, polyfunctionality score.
Following vaccination, infants and adults showed significant increases in FS (infants: 0.01 to 0.04, P = .009; adults: 0.02 to 0.09, P < .001) and PFS (infants: 0.008 to 0.02, P = .01; adults: 0.008 to 0.04, P < .001). The FS and PFS of adults post-vaccination, however, were significantly higher than those of infants (FS adjusted Δ = 0.04, P = .005; PFS adjusted Δ = 0.02, P = .01; Figure 3C).
Accentuated Induction of CD4 T-Cell Spike-Specific Responses Following mRNA-1273 vs BNT162b mRNA Vaccination in Infants
When considered by manufacturer, there was a significantly higher frequency of post-vaccine CD4 T-cells producing IL-2 (adjusted Δ = 0.14%, P = .02) or Th2 cytokines (adjusted Δ = 0.05%, P = .02) and expressing CD154 (adjusted difference, 0.22%; P = .03) among infants vaccinated with mRNA-1273 vs BNT162b. There was also a trend toward increased IL-17 production (adjusted difference, 0.03%; P = .07) but no difference in TNF-α or IFN-γ production. Infants who received mRNA-1273 vs BNT162b additionally had higher FS (adjusted difference, 0.05; P = .02) and PFS (0.03, P = .04) post-vaccination, driven by an attenuated response in the BTN162b recipients (Figure 3D). In adults, mRNA-1273 vs BNT162b recipients had a nonsignificantly higher frequency of CD4 T-cells producing IFN-γ (adjusted difference, 0.35%; P = .1), TNF-α (0.69%, P = .2), IL-2 (0.69%, P = .2), and Th2 cytokines (0.08%, P = .1) and significantly higher expression of CD154 (1.44%, P = .02). Finally, postvaccine FS (adjusted difference, 0.03; P = .1) and PFS (0.02, P = .2) were nonsignificantly increased in mRNA-1273 vs BNT162b recipients.
Infants and Adults Generate Similar Spike-Specific IgG Titers Following Vaccination
Prior work has reported a similar magnitude of anti-spike IgG following mRNA vaccination in adults and children aged 6 months to 5 years [2, 3]. Due to our observed differences in CD4 T-cell responses between adults and infants, we sought to confirm whether this was also true in our cohort. At the pre-vaccine time point, all adults were spike-specific IgG negative, whereas in infants, 5 of 13 had detectable spike-specific IgG (Figure 4A). All infants were NC-specific IgG negative at the pre-vaccine time point; thus, these titers likely reflect residual transplacentally transferred maternal IgG and not early infection. Following vaccination, all infants and adults had robust induction of spike-specific IgG (Figure 4A and 4B). Postvaccine, the mean endpoint titers among infants and adults were similar (mean, 4.67 vs 4.66 log10; adjusted difference, 0.23 log10; P = .2). There was no difference in post-vaccine titer in infants who were positive vs negative for spike-specific IgG at the pre-vaccine time point (mean, 4.53 vs 4.75 log10; P = .2). In children, there was no difference in post-vaccine spike-specific IgG titer by vaccine manufacturer (adjusted difference, −0.18 log10; P = .3), whereas among adults, the titers were significantly higher in mRNA-1273 vs BNT162b recipients (adjusted difference, 0.66 log10; P = .002). At the postvaccine time point, there was a strong correlation between the magnitude of the spike-specific T-cell FS and IgG endpoint titers in adults (R2 = 0.81, P = .001) but not infants (R2 = 0.08, P = .8; Figure 4C and 4D).
Figure 4.
Anti-spike IgG antibody endpoint titers are correlated with CD4 T-cell response following vaccination in adults but not infants. A and B, Paired anti-spike IgG endpoint titers from infants and adults before and after SARS-CoV-2 vaccination. All post-vaccine endpoint titers were above the positive threshold of log10 (50), represented as a dashed line. Infants with positive anti-spike endpoint titers prior to vaccination (red) are likely due to transplacental maternal IgG transfer. C and D, Infant and adult anti-spike IgG endpoint titers plotted against spike CD4 T-cell FS from post-vaccine time points, shown with a best-fit line. Pre- vs post-vaccine endpoint titers were compared by paired t tests. CD4 FS vs endpoint titers were compared with Pearson correlation. A value of 1:49 was used for samples negative at the initial 1:50 dilution. ***P < .001. FS, functionality score.
DISCUSSION
Respiratory viruses remain a major cause of morbidity and mortality in infants, and there is a critical need to interrogate adaptive immune responses in infants to improve the design of safe and effective vaccines. To that end, we examined the CD4 T-cell responses induced by the primary SARS-CoV-2 mRNA vaccine series in infants. We found that infants had lower frequencies of spike-specific CD4 T-cells that produced Th1 cytokines, particularly IFN-γ, relative to adults. This deficit translated to lower functionality/polyfunctionality post-vaccination relative to adults. Furthermore, the CD4 T-cell responses were higher in recipients who received mRNA-1273 vs BNT162b, although these differences were significant only in infants. In contrast to CD4 T-cell responses, spike-specific post-vaccine IgG titers were similar between infants and adults. Among infants, they were not modified by vaccine manufacturer or the presence of pre-vaccine maternal spike-specific IgG. Together, these data emphasize that infants have lower CD4 T-cell induction following SARS-CoV-2 mRNA vaccination relative to adults, with the greatest impact on IFN-γ–secreting subsets.
Infant T-cells are equipped to mount antigen-specific responses following vaccination, but their adaptive responses are altered relative to adults. T-cell function and memory response may be attenuated, which has been attributed to antigen-presenting cell immaturity [34]. Infant T-cells are highly proliferative but produce less IFN-γ and other Th1 cytokines than adult T-cells [35–37] in response to various stimuli, including infection and vaccines [38, 39]. For example, infants have been found to produce less IFN-γ in response to measles or mumps vaccination [35, 36]. Instead, immune stimuli may induce a predominant Th2 or Th17 response, although this difference has primarily been observed in young infants [37, 40, 41]. Although our observation of a deficit in CD4 T-cell production of IFN-γ among infants is consistent with these observations, we did not find evidence of a shift toward a predominant Th2 or Th17 response, similar to a recent study investigating T-cell responses to mRNA-1273 in children [19]. In contrast to infants, our adults had a robust Th1 and, to a lesser extent, Th2 response to vaccination, consistent with previously described spike-specific CD4 T-cell cytokine-producing frequencies following SARS-CoV-2 mRNA vaccination [20].
Prior work in other populations has demonstrated the importance of IFN-γ responses to protect against risk of severe COVID-19. For example, multiple studies in adults have found that low IFN-γ responses early in infection are associated with increased risk of hospitalization [42], poor outcome [43], and mortality [44], an association confirmed in experimental models [45]. In addition, the importance of IFN-γ responses to respiratory viruses such as RSV has been demonstrated in infants, where low IFN-γ responses are associated with increased risk of severe outcomes [46–48], also confirmed in experimental models [49]. Thus, while our study was not powered to look at disease outcome, our observation of restricted IFN-γ production following SARS2-CoV-2 mRNA vaccination may be a factor driving decreased efficacy in this age group [2, 3] as well as increased risk of severe disease in young infants when infected [50].
The mRNA-1273 and BNT162b mRNA vaccines are safe and effective for persons as young as 6 months of age. In this cohort, we found an accentuated response to vaccination among infants who received mRNA-1273. Although the adult response was also higher following mRNA-1273, this difference was not statistically significant. A potential explanation for the difference in CD4 T-cell responses between mRNA-1273 and BNT162b is the difference in mRNA dose between the 2 vaccines, despite the extra-dose recommendation for BNT162b in the infant series. Within adults, mRNA-1273 has been associated with higher spike-specific endpoint titers and reduced risk of severe disease, particularly among the elderly [51–53]. In contrast, no difference has been identified in spike-specific IgG induced by the 2 vaccines in children aged 6 months to 5 years [54]. Further investigation into vaccine efficacy in infants and children is needed to discern if the accentuated CD4 T-cell response generated by mRNA-1273 leads to better disease control when infected.
Our study has several limitations. First, our cohort size was relatively small, but this was offset by the ability to collect sufficient volume of blood to robustly characterize CD4 T-cell responses. Furthermore, despite the size, the cohort had limited heterogeneity, and all participants were NC-IgG negative at the pre- and post-vaccine time points, emphasizing that the observed effects were secondary to vaccination alone and not hybrid immunity. Second, infant participants had their blood drawn a median 4 weeks following the final vaccine dose, whereas adults were drawn at a median 2 weeks post-vaccine. Although the kinetics and durability of the T-cell response to SARS-CoV-2 vaccination remain understudied, particularly in children, a previous study identified stable spike-specific CD4 functionality for up to 12 months in unvaccinated children younger than 5 years following infection [55]. We additionally controlled for time post-vaccination to account for this difference. Finally, our sample size was not large enough to assess for vaccine interference, although we did not find a difference in antibody titers or magnitude of T-cell response in infants with and without maternal antibody at the time of vaccination.
Together, our findings emphasize the importance of studying CD4 T-cell responses induced by vaccination, in addition to antibody titers, where CD4 T-cell responses may be particularly important in the control of infection and the prevention of severe disease. In addition, CD4 T-cell responses may be differentially affected by age and vaccine manufacturer. Our data emphasize the need to consider immune ontogeny in the design of novel pediatric vaccines. Future studies should determine whether the observed differences in CD4 T-cell responses translate to differences in SARS-CoV-2 disease severity among vaccinated individuals.
Supplementary Material
Notes
Author contributions . M. Q. P.: conceptualization, data curation, analysis, investigation, visualization, writing–original draft, writing–review and editing; A. L. Y.: data curation, project administration, writing–review and editing; J. E. S.: data curation, project administration, writing–review and editing; M. G.: data curation, writing–review and editing; E. L.: data curation, investigation, methodology, writing–review and editing; J. K. L.: data curation, writing–review and editing; N. K. M.: data curation, writing–review and editing; H. Y. C.: data curation, writing–review and editing; J. A. E.: data curation, resources, writing–review and editing; D. N. S.: data curation, investigation, methodology, project administration, resources, writing–original draft, writing–review and editing; C. S.: methodology, project administration, resources, supervision, writing–review and editing; A. K.: conceptualization, data curation, funding acquisition, investigation, project administration, resources, supervision, writing–review and editing; W. E. H.: conceptualization, data curation, formal analysis, project administration, resources, supervision, writing–original draft, writing–review and editing.
Financial support. This work was supported by the Burroughs Wellcome Fund (BWF CAMS 1017213; to W. E. H.); Department of Pediatrics, University of Washington (to W. E. H.); Seattle Children's Research Institute (to W. E. H.); University of Washington Royalty Research Fund (to A. K.); the National Institute of Allergy and Infectious Diseases (K23 AI153390; to A. K.); and the National Center for Advancing Translational Sciences of the National Institutes of Health (UL1 TR002319).
Contributor Information
M Quinn Peters, Center for Global Infectious Disease Research, Seattle Children's Research Institute, Seattle, Washington, USA.
Amber L Young, Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, School of Medicine, University of Washington, Seattle, Washington, USA; School of Medicine, University of Colorado, Aurora, Colorado, USA.
Jennifer E Stolarczuk, Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, School of Medicine, University of Washington, Seattle, Washington, USA; School of Medicine, University of Washington, Seattle, Washington, USA.
Madeline Glad, Center for Global Infectious Disease Research, Seattle Children's Research Institute, Seattle, Washington, USA.
Erik Layton, Division of Allergy and Infectious Diseases, Department of Medicine, School of Medicine, University of Washington, Seattle, Washington, USA.
Jennifer K Logue, Division of Allergy and Infectious Diseases, Department of Medicine, School of Medicine, University of Washington, Seattle, Washington, USA.
Nana K Minkah, Center for Global Infectious Disease Research, Seattle Children's Research Institute, Seattle, Washington, USA; Division of Infectious Diseases, Department of Pediatrics, School of Medicine, University of Washington, Seattle, Washington, USA; Department of Global Health, University of Washington, Seattle, Washington, USA.
Helen Y Chu, Division of Allergy and Infectious Diseases, Department of Medicine, School of Medicine, University of Washington, Seattle, Washington, USA.
Janet A Englund, Division of Infectious Diseases, Department of Pediatrics, School of Medicine, University of Washington, Seattle, Washington, USA; Center for Clinical and Translational Research, Seattle Children's Research Institute, Seattle, Washington, USA.
D Noah Sather, Center for Global Infectious Disease Research, Seattle Children's Research Institute, Seattle, Washington, USA; Division of Infectious Diseases, Department of Pediatrics, School of Medicine, University of Washington, Seattle, Washington, USA; Department of Global Health, University of Washington, Seattle, Washington, USA.
Chetan Seshadri, Division of Allergy and Infectious Diseases, Department of Medicine, School of Medicine, University of Washington, Seattle, Washington, USA.
Alisa Kachikis, Division of Maternal Fetal Medicine, Department of Obstetrics and Gynecology, School of Medicine, University of Washington, Seattle, Washington, USA.
Whitney E Harrington, Center for Global Infectious Disease Research, Seattle Children's Research Institute, Seattle, Washington, USA; Division of Infectious Diseases, Department of Pediatrics, School of Medicine, University of Washington, Seattle, Washington, USA; Department of Global Health, University of Washington, Seattle, Washington, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Beijnen EMS, Odumade OA, Haren SDV. Molecular determinants of the early life immune response to COVID-19 infection and immunization. Vaccines (Basel) 2023; 11:590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Munoz FM, Sher LD, Sabharwal C, et al. Evaluation of BNT162b2 COVID-19 vaccine in children younger than 5 years of age. N Engl J Med 2023; 388:621–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anderson EJ, Creech CB, Berthaud V, et al. Evaluation of mRNA-1273 vaccine in children 6 months to 5 years of age. N Engl J Med 2022; 387:1673–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walter EB, Talaat KR, Sabharwal C, et al. Evaluation of the BNT162b2 COVID-19 vaccine in children 5 to 11 years of age. N Engl J Med 2022; 386:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Earle KA, Ambrosino DM, Fiore-Gartland A, et al. Evidence for antibody as a protective correlate for COVID-19 vaccines. Vaccine 2021; 39:4423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy clinical trial. Science 2022; 375:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 2020; 183:996–1012.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nelson RW, Chen Y, Venezia OL, et al. SARS-CoV-2 epitope-specific CD4(+) memory T cell responses across COVID-19 disease severity and antibody durability. Sci Immunol 2022; 7:eabl9464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bertoletti A, Le Bert N, Tan AT. SARS-CoV-2–specific T cells in the changing landscape of the COVID-19 pandemic. Immunity 2022; 55:1764–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang G, Cao J, Qin J, et al. Initial COVID-19 severity influenced by SARS-CoV-2-specific T cells imprints T-cell memory and inversely affects reinfection. Signal Transduct Target Ther 2024; 9:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Almendro-Vazquez P, Laguna-Goya R, Paz-Artal E. Defending against SARS-CoV-2: the T cell perspective. Front Immunol 2023; 14:1107803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moss P. The T cell immune response against SARS-CoV-2. Nat Immunol 2022; 23:186–93. [DOI] [PubMed] [Google Scholar]
- 15. Sette A, Crotty S. Immunological memory to SARS-CoV-2 infection and COVID-19 vaccines. Immunol Rev 2022; 310:27–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rodda LB, Morawski PA, Pruner KB, et al. Imprinted SARS-CoV-2-specific memory lymphocytes define hybrid immunity. Cell 2022; 185:1588–601.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang Z, Mateus J, Coelho CH, et al. Humoral and cellular immune memory to four COVID-19 vaccines. Cell 2022; 185:2434–51.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Painter MM, Mathew D, Goel RR, et al. Rapid induction of antigen-specific CD4(+) T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity 2021; 54:2133–42.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rostad CA, Campbell JD, Paulsen GC, et al. Evaluation of cellular immune responses after mRNA-1273 vaccination in children 6 months to 11 years of age. J Infect Dis 2025; 231:e945–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phan JM, Layton ED, Yu KKQ, et al. Cytotoxic T cells targeting spike glycoprotein are associated with hybrid immunity to SARS-CoV-2. J Immunol 2023; 210:1236–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liu J, Chandrashekar A, Sellers D, et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature 2022; 603:493–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organization . The Pfizer BioNTech (BNT162b2) COVID-19 vaccine: what you need to know. 2022. Updated 18 August 2022. Available at: https://www.who.int/news-room/feature-stories/detail/who-can-take-the-pfizer-biontech-covid-19--vaccine-what-you-need-to-know
- 23. World Health Organization . The Moderna COVID-19 (mRNA-1273) vaccine: what you need to know. 2022. Updated 18 August 2022. Available at: https://www.who.int/news-room/feature-stories/detail/the-moderna-covid-19-mrna-1273-vaccine-what-you-need-to-know
- 24. Armistead B, Jiang Y, Carlson M, et al. Spike-specific T cells are enriched in breastmilk following SARS-CoV-2 mRNA vaccination. Mucosal Immunol 2023; 16:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chu HY, Englund JA, Starita LM, et al. Early detection of COVID-19 through a citywide pandemic surveillance platform. N Engl J Med 2020; 383:185–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Harrington WE, Trakhimets O, Andrade DV, et al. Rapid decline of neutralizing antibodies is associated with decay of IgM in adults recovered from mild COVID-19. Cell Rep Med 2021; 2:100253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Files MA, Gentles L, Kehoe L, et al. Kinetics and durability of antibody and T-cell responses to SARS-CoV-2 in children. J Infect Dis 2024; 230:889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin L, Finak G, Ushey K, et al. COMPASS identifies T-cell subsets correlated with clinical outcomes. Nat Biotechnol 2015; 33:610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Balle C, Armistead B, Kiravu A, et al. Factors influencing maternal microchimerism throughout infancy and its impact on infant T cell immunity. J Clin Invest 2022; 132:e148826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rozot V, Nemes E, Geldenhuys H, et al. Multidimensional analyses reveal modulation of adaptive and innate immune subsets by tuberculosis vaccines. Commun Biol 2020; 3:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sun M, Phan JM, Kieswetter NS, et al. Specific CD4(+) T cell phenotypes associate with bacterial control in people who “resist” infection with Mycobacterium tuberculosis. Nat Immunol 2024; 25:1411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016; 32:2847–9. [DOI] [PubMed] [Google Scholar]
- 33. Mateus J, Grifoni A, Tarke A, et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 2020; 370:89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Upham JW, Rate A, Rowe J, Kusel M, Sly PD, Holt PG. Dendritic cell immaturity during infancy restricts the capacity to express vaccine-specific T-cell memory. Infect Immun 2006; 74:1106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gans H, Yasukawa L, Rinki M, et al. Immune responses to measles and mumps vaccination of infants at 6, 9, and 12 months. J Infect Dis 2001; 184:817–26. [DOI] [PubMed] [Google Scholar]
- 36. Gans HA, Maldonado Y, Yasukawa LL, et al. IL-12, IFN-gamma, and T cell proliferation to measles in immunized infants. J Immunol 1999; 162:5569–75. [PubMed] [Google Scholar]
- 37. Ota MO, Vekemans J, Schlegel-Haueter SE, et al. Hepatitis B immunisation induces higher antibody and memory Th2 responses in new-borns than in adults. Vaccine 2004; 22:511–9. [DOI] [PubMed] [Google Scholar]
- 38. Debock I, Flamand V. Unbalanced neonatal CD4(+) T-cell immunity. Front Immunol 2014; 5:393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kollmann TR, Kampmann B, Mazmanian SK, Marchant A, Levy O. Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny. Immunity 2017; 46:350–63. [DOI] [PubMed] [Google Scholar]
- 40. Kristjansson S, Bjarnarson SP, Wennergren G, et al. Respiratory syncytial virus and other respiratory viruses during the first 3 months of life promote a local TH2-like response. J Allergy Clin Immunol 2005; 116:805–11. [DOI] [PubMed] [Google Scholar]
- 41. Halonen M, Lohman IC, Stern DA, et al. Th1/Th2 patterns and balance in cytokine production in the parents and infants of a large birth cohort. J Immunol 2009; 182:3285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cremoni M, Allouche J, Graca D, et al. Low baseline IFN-gamma response could predict hospitalization in COVID-19 patients. Front Immunol 2022; 13:953502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Safont G, Villar-Hernandez R, Smalchuk D, et al. Measurement of IFN-gamma and IL-2 for the assessment of the cellular immunity against SARS-CoV-2. Sci Rep 2024; 14:1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Masia M, de la Rica A, Fernandez-Gonzalez M, et al. Integrating SARS-CoV-2-specific interferon-gamma release assay testing in the evaluation of patients hospitalized with COVID-19. Microbiol Spectr 2023; 11:e0241923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hilligan KL, Namasivayam S, Clancy CS, et al. Bacterial-induced or passively administered interferon gamma conditions the lung for early control of SARS-CoV-2. Nat Commun 2023; 14:8229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Semple MG, Dankert HM, Ebrahimi B, et al. Severe respiratory syncytial virus bronchiolitis in infants is associated with reduced airway interferon gamma and substance P. PLoS One 2007; 2:e1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garcia C, Soriano-Fallas A, Lozano J, et al. Decreased innate immune cytokine responses correlate with disease severity in children with respiratory syncytial virus and human rhinovirus bronchiolitis. Pediatr Infect Dis J 2012; 31:86–9. [DOI] [PubMed] [Google Scholar]
- 48. Bont L, Heijnen CJ, Kavelaars A, et al. Local interferon-gamma levels during respiratory syncytial virus lower respiratory tract infection are associated with disease severity. J Infect Dis 2001; 184:355–8. [DOI] [PubMed] [Google Scholar]
- 49. Lee YM, Miyahara N, Takeda K, et al. IFN-gamma production during initial infection determines the outcome of reinfection with respiratory syncytial virus. Am J Respir Crit Care Med 2008; 177:208–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Havers FP, Whitaker M, Chatwani B, et al. COVID-19–associated hospitalizations and maternal vaccination among infants aged <6 months—COVID-NET, 12 states, October 2022–April 2024. MMWR Morb Mortal Wkly Rep 2024; 73:830–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Self WH, Tenforde MW, Rhoads JP, et al. Comparative effectiveness of Moderna, Pfizer-BioNTech, and Janssen (Johnson & Johnson) vaccines in preventing COVID-19 hospitalizations among adults without immunocompromising conditions—United States, March–August 2021. MMWR Morb Mortal Wkly Rep 2021; 70:1337–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Puranik A, Lenehan PJ, Silvert E, et al. Comparative effectiveness of mRNA-1273 and BNT162b2 against symptomatic SARS-CoV-2 infection. Med 2022; 3:28–41.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Atanasov V, Barreto N, Whittle J, et al. Selection effects and COVID-19 mortality risk after Pfizer vs Moderna vaccination: evidence from linked mortality and vaccination records. Vaccines (Basel) 2023; 11:971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dalapati T, Williams CA, Giorgi EE, et al. Immunogenicity of monovalent mRNA-1273 and BNT162b2 vaccines in children <5 years of age. Pediatrics 2024; 153:e2024066190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Files MA, Gentles L, Kehoe L, et al. The kinetics and durability of antibody and T-cell responses to SARS-CoV-2 in children. J Infect Dis 2024; 230:889–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.