Abstract
Introduction
Insulin resistance (IR) is linked to several metabolic diseases including type 2 diabetes mellitus (T2DM), metabolic syndrome, and metabolic dysfunction-associated fatty liver disease (MAFD). The factors that contribute to IR in rural African populations remain largely unknown. Understanding the determinants of IR will contribute to the management of several non-communicable diseases (NCDs).
Methods
A cross-sectional study was conducted in two rural districts in northern Ghana involving male and female participants, aged 40 to 60 years, who were recruited into the study between the years 2015 and 2016. Sociodemographic, lifestyle, anthropometric, ultrasound, blood lipid profile, blood glucose and insulin, urine creatinine and urine protein data were collected. Insulin resistance was determined using the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) formula. Multivariable linear regression analyses were performed between log-transformed IR and several variables. All association analyses were considered significant at p < 0.05.
Results
The median (log-transformed) IR among women (0.54) was significantly higher than that among men (0.43) (p < 0.001). The prevalence of IR was 7.6% in the study population with more women having IR (9.9%) than men (4.5%) (p = 0.007). Drivers of IR among women were unmarried status (β = 1.19, p = 0.037), smoking (β = 8.33, P = 0.001) and triglyceride (TG) (β = 2.09, p = 0.016) while that among men were body mass index (BMI) (β = 0.47, p = 0.013), right carotid intima median thickness (CIMTright) (β = 5.08, p = 0.033), visceral adipose tissue (VAT) (β = 0.59, p = 0.031) and TG (β = 5.58, p < 0.001). Among the total population, vendor meal consumption (β = 0.41, p = 0.001), CIMTright (β = 3.54, p = 0.028), low-density lipoprotein cholesterol (LDL-C) (β = 1.08, p = 0.012), and TG (β = 2.87, p < 0.001) were linked to IR.
Conclusions
Lifestyle, adiposity, CIMTright and lipid markers contribute to driving IR levels and that these factors are gender-specific in this northern rural Ghanaian population.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-025-24806-6.
Keywords: Insulin resistance, AWI-Gen, Ghanaian, Non-communicable diseases
Introduction
Non-communicable diseases (NCDs) are the leading cause of premature death worldwide with about 85% of all annual deaths occurring in low- and middle-income countries [1]. In sub-Saharan Africa (SSA), the burden of NCD increased by 67% between 1990 and 2017 causing the increase in proportion of total disability-adjusted life years attributable to NCD from 18% to 30% [2]. Ghana like other African countries is confronted with high burden of NCDs [3, 4]. For example, a previous study in southern Ghana reported NCD prevalence of 26.7% [5]. Insulin resistance (IR) is a contributing factor of several NCDs and it has been associated with all-cause mortality at a rate of 20.6% to 25.3% globally [6]. Insulin Resistance (IR) is a condition in which the body cells become less responsive to insulin and this contributes to abnormal or reduced physiological activity [7]. Insulin resistance causes dysregulation of glucose metabolism that results in oxidative stress, prolonged hyperglycemia, and inflammatory reactions that harm cells [8]. Insulin resistance is further linked with decreased efficiency to utilize fatty acids as an energy source in skeletal muscle and liver tissues [9]. Insulin resistance has been linked to several diseases including type 2 diabetes mellitus (T2DM), metabolic syndrome, metabolic dysfunction-associated fatty liver disease (MAFD), Alzheimer’s disease and cancer [10–13]. Therefore, understanding the factors that drive IR will contribute to the control of several NCDs including cancers, cardiometabolic, and neurologic diseases.
Though several studies in non-continental Africa have linked IR to risk factors including body fat distribution (BMI, VAT, SAT, HC and WC) [14], metabolic factors (menopausal status [15], socioeconomic (age, sex, SES) [16, 17] and lifestyle factors (unhealthy dietary patterns, physical activity, smoke and alcohol intake) [18], there is a paucity of data on IR and the driving factors in SSA in general and Ghana in particular. The prevalence of IR-associated risk factors is increasing in Ghana. For instance, it has been reported that the prevalence of overweight tripled and obesity doubled between 1993 and 2014 in the country [19]. Similarly, it is reported that overweight increased from 15.5% in 2017/2018 to 33.2% in 2019/2020 while obesity increased from 8.6% to 16.8% within the same period [20]. Furthermore, studies have observed poor dietary habits and sedentary lifestyle among Ghanaians [21, 22]. Despite the rising prevalence of these risk factors no population-based study in Ghana has investigated the influence of an array of factors including sociodemographic, lifestyle, anthropometry, lipids and body adiposity on IR. Though a previous study in urban northern Ghana reported insulin resistance prevalence of 61% in adults without history of COVID-19 infection, factors associated with IR were not investigated [23]. This calls for an investigation into the drivers of IR in northern Ghana. Therefore, our study determined factors associated with IR in an apparently healthy adult population in rural northern Ghana.
Methods
Study population
This was a cross-sectional study that was conducted in the Kassena-Nankana East Municipality and Kassena-Nankana West District as part of the broader Human Heredity and Health in Africa (H3Africa) Africa Wits-INDEPTH (International Network for the Demographic Evaluation of Populations and Their Health) Partnership for Genomic Studies (AWI-Gen) [24]. Study participants were recruited between the years 2015 and 2016. Men and women aged 40 to 60 years who resided in the study area for at least 10 years were included in the study. Pregnant women and individuals whose anthropometry could not be taken accurately due to physical limitations were excluded [24].
Sample size determination
The minimal sample size of 365 participants was determined using the Cochran formula n = z2p(1-p)/e [25], where n = minimum sample size, z = confidence limit at 95%=1.96, e = margin of error = 0.05, p = prevalence of insulin resistance. The sample size calculation was based on the IR prevalence of 61% in a previous study involving participants in the northern region of Ghana [23].
Data collection
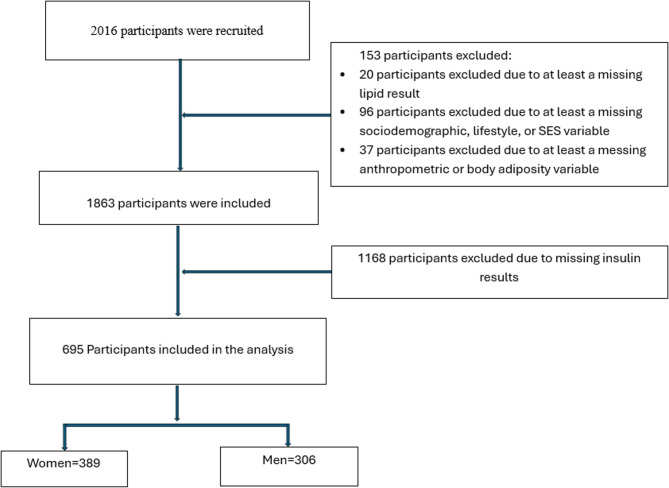
The data collection procedures employed in this study are adapted from the methods used in phase one of the AWI-Gen study [26, 27]. Data on sociodemographic and lifestyle variables were collected using a structured questionnaire. Briefly, a person who reported to have attended at least primary education was considered to have formal education. A person who was staying with a partner for at least a year during the period of recruitment was considered currently married. A participant who reported smoking or taking alcohol and had not stopped prior to the study was considered to be currently smoking or taking alcohol, respectively. Socioeconomic status (SES) was determined using the INDEPTH Health Equity tool, which is an asset index generated by using principal component analysis to combine data on household possessions [28]. The household assets were broken down into categorical variables and further converted into weights and principal components. The weights of the first principal component were used to develop an index from which scores of SES of the households were derived. These scores were divided into five quintiles. Low SES was defined as the first and second quintiles, while the third to fifth quintiles were considered high SES. Physical activity was assessed using the Global Physical Activity Questionnaire (GPAQ) [29] and measured as the number of minutes spent per week in moderate- and vigorous-intensity physical activity (MVPA). The MVPA was calculated using the World Health Organization (WHO) guidelines [30]. Those with MVPA < 150 min per week were considered low physically active and those between ≥ 150 and ≤ 250 min per week were considered to be normal in physical activity. Participants with MVPA >250 min per week were considered to have high physical activity. Pesticide exposure was defined as self-reported current use of pesticides or living close to a farm where pesticides were being used. Vendor meal consumption was defined as the number of times a meal was consumed by the participant per week from a meal vendor. Menopausal status was put into three categories. Pre-menopausal status was defined as having regular periods. Peri-menopausal status was defined as having irregular periods within the past 12 months. Post-menopausal status was defined as having no periods within the past 12 months [31]. Waist and hip circumference were measured using an elastic tape measure (SECA, Hamburg, Germany) and read to the nearest millimetre. Height was measured using a wall-mounted stadiometer (Holtain, Wales, UK). Weight was measured using an electronic weighing scale (Kendon Medical, South Africa) and read to the nearest 0.1 Kg. Body mass index (BMI) was calculated in Kg/m2 using body weight and height. The BMI was categorized as underweight: <18.5 kg/m2, normal weight: 18.5–24.9 kg/m2, overweight: 25.0–29.9 kg/m2 and obese: ≥30 kg/m2 according to World Health Organisation (WHO) recommendation [32]. Physical activity was assessed using the Global Physical Activity Questionnaire (GPAQ) [29]. Visceral adipose tissue (VAT), subcutaneous adipose tissue (SAT), and carotid intima-media thickness (CIMT) were all measured using a LOGIQ e (GE Healthcare, CT, USA) ultrasound equipment. Blood pressure (systolic and diastolic) was measured using a digital sphygmomanometer (Omron M6, Omron, Kyoto, Japan). Fasting blood glucose and blood lipids (high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), total cholesterol (TC), and triglyceride (TG) levels were measured in mmol/l using an automated chemistry analyser (Randox RX Daytona+, Crumlin, Northern Ireland). The fasting insulin levels were determined using the Immulite 1000 chemistry analysis system (Siemens, Germany) while urine biomarkers (urine albumin, urine protein, and urine creatinine) were measured using a Roche/Hitachi Cobas C501 System. The number of participants included in this study is shown in Fig. 1.
Fig. 1.
Flow diagram showing the number of study participants excluded from the data analysis and the reasons for exclusion
Determination of insulin resistance
Insulin resistance (IR) was determined using the Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) formula which is defined as (insulin (mU/ml) x glucose (mmol/L)/22.5) [33]. Insulin resistance was defined as HOMA-IR >2.5 [34]. The HOMA-IR method is simple, less costly and compared favourably with other methods for assessment of IR [35].
Statistical analyses
The data was analysed using STATA 17.0 (StataCorp, College Station, Texas, 77845, US). Normality of data was assessed using the Shapiro-Wilk Test. Continuous variables that were skewed were presented as medians and interquartile ranges while those that were normally distributed were presented as mean (± standard deviation). Categorical variables were presented as proportions (%). Mann-Whitney U test was used to compare skewed variables between men and women while Student t-test was used to compare non-skewed variables between the two groups. Pearson’s χ2 test was used to compare categorical variables between the groups. The IR variable was log-transformed before sex-stratified linear regression analyses were performed to determine the factors associated with IR. Variables that correlated with logIR in the univariate linear regression analysis at a significance level of p < 0.20 were included in the multivariable linear regression analysis. Variance Inflation Factor (VIF) was used to assess collinearity among variables and those with VIF < 5.0 were included in the models. All tests at the multivariable linear regression models were considered significant at P < 0.05.
Results
Sociodemographic, lifestyle, and biomarker profile of the study population
The sociodemographic and lifestyle characteristics of the study population are presented in Table 1. The proportion of individuals with formal education was low (26.9%), with more men being formally educated than women (P < 0.001). Similarly, a greater proportion of the study participants was married, with men constituting the higher percentage of this group (P < 0.001). Current alcohol consumption and smoking were higher among men than women (p < 0.001 for both). The population was physically active (84.9%), with men being more physically active than women (P < 0.001). Socioeconomic status (SES) of men was higher(P = 0.029) and men were more exposed to pesticides (P = 0.001) than women. Additionally, the number of vegetables (P = 0.00) and vendor meals consumed per week (P < 0.001) was greater among men than women (P = 0.002). The prevalence of obesity in the study population was low (4.0%), with women being more obese than men (P < 0.001). The women were predominantly at their peri- and post-menopausal state (62.6%).
Table 1.
Sociodemographic and lifestyle characteristics of the study population stratified by sex
| Variable | Men (N = 306) | Women (N = 389) | Total (N = 695) | P value* | |||
|---|---|---|---|---|---|---|---|
| Educational Status | |||||||
| No Formal Education | 198 (64.7) | 310 (79.7) | 508 (73.1) | ||||
| Formal Education | 108 (35.3) | 79 (20.3) | 187 (26.9) | < 0.001 | |||
| Marital Status | |||||||
| Currently Unmarried | 48 (15.7) | 150 (38.6) | 198 (28.5) | ||||
| Currently Married | 258 (84.3) | 239 (61.4) | 497 (71.5) | < 0.001 | |||
| Alcohol Intake | |||||||
| No Current Intake | 27 (8.8) | 84 (21.6) | 111 (16.0) | ||||
| Current Intake | 279 (91.2) | 305 (78.4) | 584 (84.0) | < 0.001 | |||
| Smoking Status | |||||||
| No current Smoking | 104 (34.0) | 384 (98.7) | 488 (70.2) | ||||
| Current Smoking | 202 (66.0) | 5 (1.3) | 207 (29.8) | < 0.001 | |||
| Physical Activity | |||||||
| Low | 16 (5.2) | 56 (14.4) | 72 (10.4) | ||||
| Normal | 9 (2.9) | 9 (2.9) | 33 (4.8) | ||||
| High | 281 (91.8) | 309 (79.4) | 590 (84.9) | < 0.001 | |||
| SES | |||||||
| Low | 217 (70.9) | 304 (78.2) | 521 (75.0) | ||||
| High | 89 (29.1) | 85 (21.8) | 174 (25.0) | 0.029 | |||
| Pesticide Exposure | |||||||
| No Exposed | 111 (36.3) | 188 (48.3) | 299 (43.0) | ||||
| Exposed | 195 (63.7) | 201 (51.7) | 396 (57.0) | 0.001 | |||
| BMI Categories | |||||||
| Underweight | 52 (17.0) | 34 (8.8) | 86 (12.4) | ||||
| Normal | 232 (75.8) | 260 (66.8) | 492 (70.8) | ||||
| Overweight | 19 (6.2) | 70 (18.0) | 89 (12.8) | < 0.001 | |||
| Obese | 3 (1.0) | 25 (6.4) | 28 (4.0) | ||||
| Menopausal status | |||||||
| Pre-menopausal | 237 (37.4) | 237 (37.4) | |||||
| Peri-menopausal | 245 (24.7) | 245 (24.7) | |||||
| Post-menopausal | 376 (37.9) | 376 (37.9) | |||||
| Fruit Servings/per week | 1.0 (± 1.6) | 1.0 (± 1.7) | 1.1 (± 1.7) | 0.293 | |||
| Vegetable Servings/per week | 3.4 (± 1.5) | 3.2 (± 1.5) | 3.3 (± 1.5) | 0.001 | |||
| Vendor Meals/per week | 1.2 (± 1.7) | 0.8 (± 1.3) | 1.0 (± 1.5) | < 0.001 | |||
*P value for the difference between men and women, χ2: Chi square, SES: Socioeconomic status, fruit, and vegetable servings, and vendor meal are measured in mean (± standard deviation)
Women had higher BMI (P < 0.001), HC (P < 0.001), SAT thickness (P < 0.001), insulin (P < 0.001), and glucose levels (P = 0.004) than men. On the other hand, men had higher WC (P < 0.001), and VAT thickness (P < 0.001) than women (Table 2).
Table 2.
Biomarker profile of the study population stratified by sex
| Variable | Men (N = 306) | Women (N = 389) | Total (N = 695) | P value* |
|---|---|---|---|---|
| BMI (kg/m2) | 20.6 (15.6, 33.3) | 22.0 (15.1, 43.1) | 21.3 (15.1, 43.1) | < 0.001 |
| WC (cm) | 72.5 (56.0, 100.1) | 75.0 (58.0, 110.7) | 74.0 (56.0, 110.7) | < 0.001 |
| HC (cm) | 82.8 (70.0, 107.0) | 88.0 (11.9, 134.0) | 86.0 (11.9, 134.0) | < 0.001 |
| WHR | 0.9 (0.8, 1.1) | 0.9 (0.6, 8.2) | 0.9 (0.6, 8.2) | < 0.001 |
| SBP (mmHg) | 121.5 (81.5, 204.0) | 120.5 (89.0, 221.0) | 121.0 (81.5, 221.0) | 0.537 |
| DBP (mmHg) | 74.5 (50.0, 130.0) | 76.0 (53.0, 129.5) | 75.5 (50.0, 130.0) | 0.369 |
| CIMTLeft (cm) | 0.7 (0.4, 6.7) | 0.7 (0.4, 6.7) | 0.7 (0.1, 6.7) | 0.217 |
| CIMTRight (cm) | 0.7 (0.1, 1.0) | 0.7 (0.1, 1.1) | 0.7 (0.1, 1.1) | 0.662 |
| Insulin (mIU/ml) | 2.0 (2.0, 301.0) | 2.6 (2.0, 301.0) | 2.0 (2.0, 301.0) | < 0.001 |
| Glucose (mmol/l) | 4.3 (2.2, 11.9) | 4.5 (3.1, 20.7) | 4.4 (2.2, 20.7) | 0.004 |
| HDL-C (mmol/l) | 1.2 (0.1, 3.0) | 1.2 (0.2, 2.6) | 1.2 (0.1, 3.0) | 0.274 |
| LDL-C (mmol/l) | 1.5 (0.4, 5.4) | 1.5 (0.4, 5.1) | 1.5 (0.4, 5.4) | 0.664 |
| TC (mmol/l) | 3.4 (0.3, 6.6) | 3.4 (0.5, 6.8) | 3.4 (0.3, 6.8) | 0.980 |
| TG (mmol/l) | 0.6 (0.2, 2.4) | 0.6 (0.1, 2.8) | 0.6 (0.1, 2.8) | 0.137 |
| SAT (cm) | 0.7 (0.2, 2.2) | 1.0 (0.3, 3.9) | 0.8 (0.2, 3.9) | < 0.001 |
| VAT (cm) | 4.1 (1.9, 9.1) | 3.3 (1.5, 8.5) | 3.6 (1.5, 9.1) | < 0.001 |
| Urine Protein (mmol/l) | 0.1 (0.0, 3.6) | 0.1 (0.0, 7.0) | 0.1 (0.0, 7.0) | 0.036 |
| Urine Creatinine (mmol/l) | 9.7 (1.1, 41.3) | 8.4 (1.2, 35.2) | 9.1 (1.1, 41.3) | 0.067 |
*P value for the difference between men and women
WC waist circumference, HC hip circumference, WHR waist-to-hip ratio, SBP systolic blood pressure, DBP diastolic blood pressure, CIMT carotid intima median thickness, HDL-C High density lipoprotein cholesterol, LDL-C low density lipoprotein cholesterol, TC total protein, TG triglycerides SAT subcutaneous adipose tissue, VAT visceral adipose tissue
Prevalence of high IR and median IR in the study population
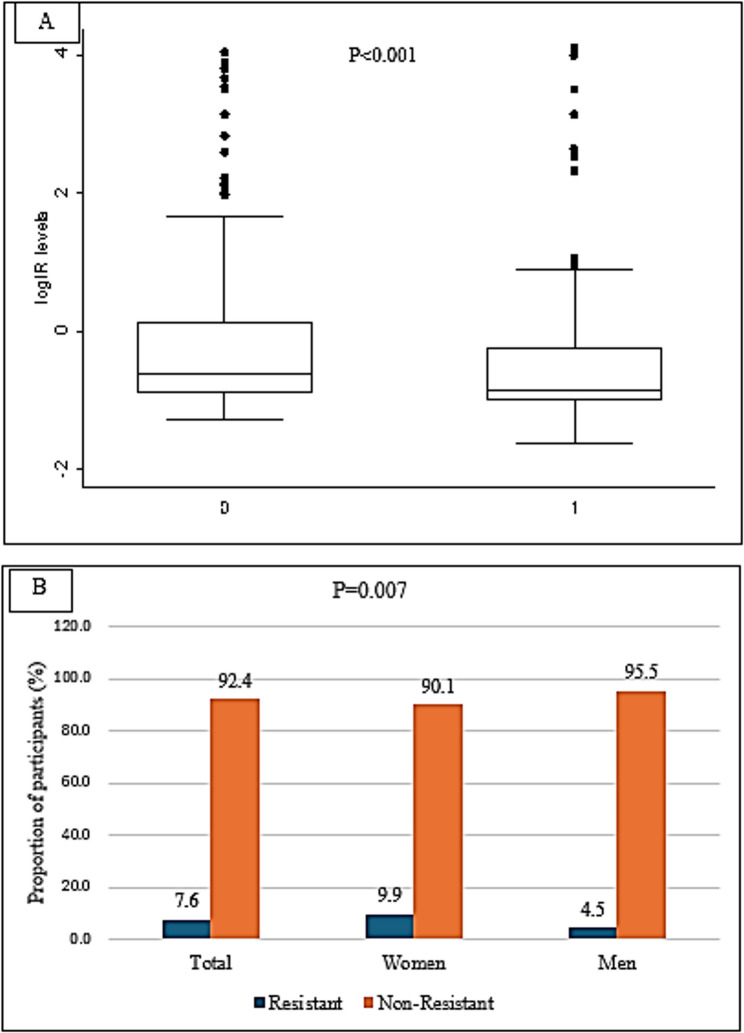
The median IR among women (0.54) was significantly higher than that among men (0.43) (P < 0.001) (Fig. 2A). The prevalence of IR in the study population (Fig. 2B) was 7.6%, with the prevalence among men (4.5%) significantly lower than that among women (9.9%) (P = 0.007).
Fig. 2.
A The median Insulin resistance levels among the study participants stratified by sex. 0: Women, 1: Men. B The proportion of insulin-resistant participants stratified by sex
Linear regression analyses of factors associated with IR among study participants
Table 3 shows the factors associated with increased IR among women in the multivariable regression models. Being unmarried (P = 0.007), current smoking (P = 0.001) and higher TG levels (P = 0.016) were all associated with IR. These factors accounted for 7.8% of the variance in IR among women.
Table 3.
Factors associated with insulin resistance among women in Northern Ghana (N = 389)
| Variable | Univariate Model | Multivariate Model | ||
|---|---|---|---|---|
| β-Coefficient (95%C.I) | P value | β-Coefficient (95%C.I) | P value | |
| Currently unmarried | 1.11 (−0.03, 2.25) | 0.055 | 1.19 (0.08, 2.30) | 0.037 |
| Currently smoking | 7.48 (2.60, 12.35) | 0.003 | 8.33 (3.59, 13.08) | 0.001 |
| BMI (Kg/m2) | 0.15 (0.02, 0.28) | 0.028 | 0.23 (−0.05, 0.50) | 0.105 |
| WC (cm) | 0.38 (−0.16, 0.92) | 0.167 | −0.48 (−1.52, 0.56) | 0.361 |
| SAT (cm) | 0.72 (−0.23, 1.66) | 0.135 | −0.53 (−1.96, 0.90) | 0.469 |
| LDL-C (mmol/l) | 0.72 (−0.10, 1.52) | 0.084 | 0.52 (−0.70, 1.74) | 0.403 |
| TC (mmol/l) | −0.53 (−1.04, 10.02) | 0.043 | −0.60 (−1.27, 0.08) | 0.081 |
| TG (mmol/l) | 2.78 (1.52, 4.04) | < 0.001 | 2.09 (0.39, 3.79) | 0.016 |
BMI Body mass index, WC Waist circumference, SAT Subcutaneous adipose tissue thickness, LDL-C low density lipoprotein cholesterol, TC total cholesterol, TG Triglycerides, C.I confidence interval
Among men, factors associated with increased IR were higher levels of BMI (P = 0.013), CIMTright (P = 0.033), VAT, and TG in the multivariable regression analysis (P < 0.001) (Table 4). These factors explained 29% of the variance in IR.
Table 4.
Factors associated with insulin resistance among men in Northern Ghana (N = 306)
| Variable | Univariate Model | Multivariate Model | ||
|---|---|---|---|---|
| β-Coefficient (95%C.I) | P value | β-Coefficient (95%C.I) | P value | |
| Vegetable servings/week | 0.27 (−1.04, 0.02) | 0.058 | −0.17 (−0.63, 0.29) | 0.457 |
| Vendor meals/week | 0.69 (0.36, 1.03) | < 0.001 | 0.29 (−0.03, 0.60) | 0.077 |
| BMI (Kg/m2) | 0.38 (0.14, 0.63) | 0.002 | 0.47 (0.10, 0.83) | 0.013 |
| WC (cm) | 1.01 (0.06, 1.97) | 0.038 | −1.62 (−3.37, 0.12) | 0.068 |
| HC (cm) | 1.17 (0.23, 2.11) | 0.015 | 0.34 (−1.42, 2.09) | 0.707 |
| CIMTright (cm) | 6.37 (0.99, 11.75) | 0.020 | 5.08 (0.42, 9.75) | 0.033 |
| VAT (cm) | 1.12 (0.58, 1.67) | < 0.001 | 0.59 (0.05, 1.12) | 0.031 |
| SAT (cm) | 2.39 (0.45, 4.34) | 0.016 | 0.29 (−1.78, 2.36) | 0.782 |
| TC (mmol/l) | −1.91 (−2.56, −1.26) | < 0.001 | −0.97 (−2.32, 0.13) | 0.072 |
| TG (mmol/l) | 6.69 (4.96, 8.41) | < 0.001 | 5.58 (3.93, 7.22) | < 0.001 |
BMI Body mass index, WC Waist circumference, SAT Subcutaneous adipose tissue thickness, LDL-C low density lipoprotein cholesterol, TC total cholesterol, TG Triglycerides, C.I confidence interval
Factors associated with insulin IR in the total population were higher vendor meal consumption per week (P = 0.001), increased levels of CIMTright (P = 0.028), LDL-C (P = 0.012), and TG (P < 0.001). These factors explained 14% of the variance in IR within the total population. (Table 5).
Table 5.
Factors associated with insulin resistance among the study total population in Northern Ghana (N = 695)
| Variable | Univariate Model | Multivariate Model | ||
|---|---|---|---|---|
| β-Coefficient (95%C.I) | P value | β-Coefficient (95%C.I) | P value | |
| Pesticide use | 0.58 (−0.30, 1.46) | 0.194 | 0.44 (−0.39, 1.28) | 0.297 |
| Vegetable servings/week | 0.34 (−0.65, −0.02) | 0.035 | −0.17 (−0.47, 0.13) | 0.261 |
| Vendor meals/week | 0.50 (0.25, 0.75) | < 0.001 | 0.41 (0.17, 0.66) | 0.001 |
| BMI (Kg/m2) | 0.20 (0.09, 0.32) | 0.001 | 0.23 (−0.001, 0.46) | 0.050 |
| WC (cm) | 0.55 (0.09, 1.02) | 0.020 | −0.60 (−1.52, 0.33) | 0.208 |
| HC (cm) | 0.35 (−0.09, 0.79) | 0.120 | −0.26 (−0.99, 0.47) | 0.479 |
| CIMTright (cm) | 3.08 (−0.29, 6.45) | 0.073 | 3.54 (0.38, 6.69) | 0.028 |
| VAT (cm) | 0.18 (0.21, 0.91) | 0.002 | 0.06 (−0.30, 0.43) | 0.736 |
| SAT (cm) | 0.99 (0.19, 1.79) | 0.015 | 0.02 (−1.10, 1.13) | 0.976 |
| LDL-C (mmol/l) | 0.66 (0.38, 1.27) | 0.037 | 1.08 (0.24, 1.92) | 0.012 |
| TC (mmol/l) | −1.10 (−1.51, −5.24) | < 0.001 | −0.09 (−1.87, 0.85) | 0.078 |
| TG (mmol/l) | 4.21 (3.18, 5.24) | < 0.001 | 2.87 (1.64, 4.09) | < 0.001 |
BMI Body mass index, WC Waist circumference, SAT Subcutaneous adipose tissue thickness, LDL-C low density lipoprotein cholesterol, TC total cholesterol, TG Triglycerides, C.I confidence interval
Discussion
Our study examined the prevalence of insulin resistance (IR) and explored the associated factors among adult men and women in northern Ghana. The findings indicated that the median IR level among women was higher than that among men, with 9.9% of women and 4.5% of men having IR. The overall proportion of the population with IR was 7.6%. These results demonstrate that IR within this population is generally low, contrary to earlier studies conducted in other African populations that reported higher prevalence of IR. In Ghana, specifically in the northern region, a prior study revealed a higher prevalence of 61%, among hospital attendees [36]. That study differed from ours, which was population-based, more rural, and had a relatively large sample size. The low level of insulin resistance (IR) in our study population could partly be attributed to their physically active lifestyle, combined with a very low prevalence of obesity. Physical inactivity and obesity both promote insulin resistance [37]. The study showed that IR was associated with VAT and BMI. Consistent with our findings, recent studies showed that VAT and BMI were predictors of IR [38, 39]. Visceral adipose tissue releases adipokines and cytokines which reduce insulin sensitivity in the liver and muscle [40] and this partly could explain the driving effect of VAT on IR. Similarly, the observed association of increased BMI with IR among men in our study could be explained by the observed influence levels of IR by VAT. Similar to VAT, high BMI contributes to IR through the release of inflammatory cytokines and non-esterified fatty acids by the adipose tissue resulting in reduced insulin sensitivity [41]. The higher levels of IR among women compared to men may partly be attributed to the greater proportion of women (62.6%) being at their peri- and post-menopausal state. Women in the premenopausal state have lower insulin resistance than men, but this diminishes as the women transition through peri-menopause to menopause. The transition from premenopausal to menopausal state is associated with decreased estrogen levels, which increases insulin resistance among women [15]. The TG and LDL-C levels in this population (TG: 0.6 mmol/l, LDL-C: 1.5mmol/l) are lower than those in urban settings in Ghana (TG: 1.5 to 4.3 mmol/l, LDL-C: 3.4 to 6.3 mmol/l) [42, 43]. This could account for the lower IR seen in this rural Ghanaian population.
The positive association observed in our study between IR and levels of TG and LDL-C aligns with several findings [44–46]. It is postulated that elevated lipid levels directly influence peripheral tissue insulin signaling, leading to the development of insulin resistance [47]. The study showed that IR was associated with CIMT, similar to results in other populations [48, 49]. Increased CIMT is an independent predictor of atherosclerosis [50, 51]. Therefore, high IR may reflect partly an underlying atherosclerotic condition in this rural Ghanaian population.
Our findings demonstrated that being unmarried was linked to increased insulin resistance (IR). To understand the factors driving the higher IR among unmarried women compared to married women we performed subgroup analysis among married and unmarried women. The findings showed that higher BMI levels among the unmarried women accounted for the difference in IR level. The association of vendor meal consumption with IR in our study is consistent with several reports which show the risk of increased IR with unhealthy dietary habits [52–55].
The study has shown that lifestyle, adiposity, atherosclerotic factors, and lipid markers contribute to increasing insulin resistance levels, and that these factors are specific to gender. Therefore, this study may contribute to the implementation of gender-specific strategies that are needed for the reduction of insulin resistance among this northern rural Ghanaian population.
Our study is significant because it is one of the few investigations in SSA that has identified IR and its driving factors within a rural adult population. We explored the association of numerous factors, including demographic, socioeconomic, lifestyle, adiposity, lipid, and urinary biomarkers with IR. However, the study has its limitations. It is a cross-sectional analysis that fails to establish causality. We recommend that further longitudinal studies be conducted to determine causality in this rural African population. We did not examine the influence of genetic factors on IR, as genetic and non-genetic factors may play a role in determining IR levels. Another limitation is that we did not evaluate the sensitivity of using HOMA-IR in assessing IR within our population, since HOMA-IR sensitivity differs across various populations [56]. Furthermore, the inability to measure estrogen levels constrains our findings, particularly concerning our female cohort. Despite these limitations, our exploration of various factors has enhanced our understanding of the determinants of IR in this population.
Supplementary Information
Acknowledgements
The authors are very grateful to all the study participants who provided their data and samples for this study. We acknowledge the support of the director and management of the Navrongo Health Research Centre (NHRC), the entire AWI-Gen team, and the laboratory staff at the NHRC, for participant recruitment, data, and sample collection. We acknowledge the Sydney Brenner Institute for Molecular Bioscience (SBIMB) team for playing lead roles in the processing of the samples and analyses.
Abbreviations
- AWI-Gen
Africa Wits-INDEPTH partnership for Genomic studies
- ACR
Urine albumin-creatinine ratio
- BMI
Body mass index
- CIMT
Carotid intima-media thickness
- DBP
Diastolic blood pressure
- H3Africa
Human Heredity and Health in Africa
- HC
Hip circumference
- HDL-C
High-density lipoprotein cholesterol
- HIV
Human immuno-deficiency virus
- HOMA-IR
Homeostasis model assessment of insulin resistance
- GPAQ
Global physical activity questionnaire
- INDEPTH
International network for the demographic evaluation of populations and their health
- LDL-C
Low-density lipoprotein cholesterol
- IR
Insulin resistance
- MAFD
Metabolic dysfunction-associated fatty liver disease
- NCDs
Non-communicable diseases
- SAT
Subcutaneous adipose tissue
- SBP
Systolic blood pressure
- SSA
Sub-Saharan Africa
- SES
Socioeconomic status
- TC
Total cholesterol
- T2DM
Type 2 diabetes mellitus
- TG
Triglycerides
- VAT
Visceral adipose tissue
- WC
Waist circumference
- WHR
Waist-to-hip ratio
Authors’ contributions
M.T., E.K.O., and G.A. conceived the idea. G.A., E.A.N., M.T. and P.A. collected the data. M.T. and G.A. wrote the first draft. G.A. and W.D. performed the data analysis. Supervision was done by G.A. and E.K.O. All authors read and approved the final draft.
Funding
The data for this study was funded by the National Institutes of Health (NIH) through the H3Africa AWI-Gen project (NIH grant number U54HG006938).
Data availability
All data generated or analysed during this study are included in the supplementary files of this published article.
Declarations
Ethics approval and consent to participate
Ethics approval was obtained from the Navrongo Health Research Centre Institutional Review Board (IRB No: NHRCIRB178). Individual written informed consent was obtained from each participant prior to their recruitment into the study. All study procedures were conducted in accordance with the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1. UNFPA. Responding to the challenge of Non-communicable diseases. UNAIDS. 2019;6.
- 2.Barry A, Impouma B, Wolfe CM, Campos A, Richards NC, Kalu A et al. Non-communicable diseases in the WHO African region: analysis of risk factors, mortality, and responses based on WHO data. Sci Rep. 2025;15(1) , 12288. 10.1038/s41598-025-97180-3 [DOI] [PMC free article] [PubMed]
- 3.Read UM, Doku VCK, Read UM. Number 2 Supplement GHANA MEDICAL JOURNAL. Ghana Med J [Internet]. 2012;46(2):29–38. Available from: http://www.globalizationandhealth.com/series.%0Ahttps://www.ajol.info/index.php/gmj/article/view/88737
- 4.Bosu WK. A comprehensive review of the policy and programmatic response to chronic non-communicable disease in Ghana. Ghana Med J. 2012;46(2 Suppl):69–78. [PMC free article] [PubMed] [Google Scholar]
- 5.Boakye H, Atabila A, Hinneh T, Ackah M, Ojo-Benys F, Bello AI. The prevalence and determinants of noncommunicable diseases among Ghanaian adults: A survey at a secondary healthcare level. PLoS One [Internet]. 2023;18(2 February):1–14. Available from: 10.1371/journal.pone.0281310 [DOI] [PMC free article] [PubMed]
- 6.Penno G, Solini A, Orsi E, Bonora E, Fondelli C, Trevisan R, et al. Insulin resistance, diabetic kidney disease, and all-cause mortality in individuals with type 2 diabetes: a prospective cohort study. BMC Med. 2021;19(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azeez TA, Osundina M. Insulin resistance and non-alcoholic fatty liver disease: a review of the pathophysiology and the potential targets for drug actions. J Thee Med Sci (Berkala Ilmu Kedokteran). 2020;52(4):365–76. [Google Scholar]
- 8.Yaribeygi H, Sathyapalan T, Atkin SL, Sahebkar A. Molecular Mechanisms Linking Oxidative Stress and Diabetes Mellitus. Oxid Med Cell Longev. 2020;2020. [DOI] [PMC free article] [PubMed]
- 9.Gilbert M. Role of skeletal muscle lipids in the pathogenesis of insulin resistance of obesity and type 2 diabetes. J Diabetes Investig. 2021;12(11):1934–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;1–8. [DOI] [PMC free article] [PubMed]
- 11.Alzheimer 's Association. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2021;17(3):327–406. [DOI] [PubMed]
- 12.Park JH. Insulin resistance in non-alcoholic fatty liver disease. Korean J Hepatol. 2006;12(1):16–30. [PubMed] [Google Scholar]
- 13.Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10(5):293–302. [DOI] [PubMed] [Google Scholar]
- 14.Raji A, Seely EW, Arky RA, Simonson DC. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab. 2001;86(11):5366–71. [DOI] [PubMed] [Google Scholar]
- 15.Genazzani A, Petrillo T, Semprini E, Aio C, Foshci M, Ambrosetti F, et al. Metabolic syndrome, insulin resistance andmenopause: the changes in body structure andthe therapeutic approach. Gynecol Reprod Endocrinol Metab. 2023;4(2–3):86–91. [Google Scholar]
- 16.Bockarie AS, Derkyi-Kwarteng L, Obeng JA, Adatsi RK, Aniakwaa-Bonsu E, Apprey C et al. Prevalence and determinants of insulin resistance in recovered COVID-19 and uninfected residents of two regional capitals in Ghana: An observational study. PLOS Glob Public Heal [Internet]. 2025;5(4 April):1–23. Available from: 10.1371/journal.pgph.0004506 [DOI] [PMC free article] [PubMed]
- 17.Riccio A, Fortin E, Mellbin L, Norhammar A, Näsman P, Rydén L, et al. Sex differences in the association between insulin resistance and non-fatal myocardial infarction across glycaemic States. Cardiovasc Diabetol. 2024;23(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oyewole Babalola O, Olamide Ottu P, Akinnusi E, Olayinka Aturamu P, Iwaloye O. Lifestyle Interventions to Manage Insulin Resistance. Glucose Insul Homeost. 2024;1–20.
- 19.Amugsi DA, Dimbuene ZT, Mberu B, Muthuri S, Ezeh AC. Prevalence and time trends in overweight and obesity among urban women: an analysis of demographic and health surveys data from 24 African countries, 1991–2014. BMJ Open. 2017;7(10). [DOI] [PMC free article] [PubMed]
- 20.Yussif MT, Morrison AE, Annan RA. 10-year level, trends and socio-demographic disparities of obesity among Ghanaian adults -A systematic review and meta-analysis of observational studies [Internet]. Vol. 4, PLOS Global Public Health. 2024. 1–36 p. Available from: 10.1371/journal.pgph.0002844 [DOI] [PMC free article] [PubMed]
- 21.Kushitor SB, Alangea DO, Aryeetey R, de-Graft Aikins A. Dietary patterns among adults in three lowincome urban communities in Accra, Ghana. PLoS One [Internet]. 2023;18(11 November):1–19. Available from: 10.1371/journal.pone.0293726 [DOI] [PMC free article] [PubMed]
- 22.Akoto S, Tandoh MA, Nsiah K, Asamoah-Boakye O, Annaful VT. Lifestyle habits, macronutrient intake, and obesity prevalence among adolescents in rural-periurban community senior high schools in the Ho municipality of Ghana. Front Nutr. 2022;9(August):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adatsi RK, Bockarie AS, Derkyi-Kwarteng L, Pappoe F, Nsiah P, Dankwa K et al. Increased insulin resistance with reduced beta cell function in recovered COVID-19 Ghanaians. Endocr Metab Sci [Internet]. 2023;13(October):100150. Available from: 10.1016/j.endmts.2023.100150
- 24.Ramsay M, Crowther N, Tambo E, Agongo G, Baloyi V, Dikotope S, et al. H3Africa AWI-Gen collaborative centre: a resource to study the interplay between genomic and environmental risk factors for cardiometabolic diseases in four sub-Saharan African countries. Glob Health Epidemiol Genom. 2016. 10.1017/gheg.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cochran W, Cochran W. Sampling techniques. 3rd ed. New York: Wiley; 1977. 1977;1977. [Google Scholar]
- 26.Ali SA, Soo C, Agongo G, Alberts M, Amenga-Etego L, Boua RP et al. Genomic and environmental risk factors for cardiometabolic diseases in Africa: methods used for Phase 1 of the AWI-Gen population cross-sectional study. Glob Health Action [Internet]. 2018;11(sup2). Available from: 10.1080/16549716.2018.1507133 [DOI] [PMC free article] [PubMed]
- 27.Agongo G, Nonterah EA, Debpuur C, Amenga-Etego L, Ali S, Oduro A et al. Correction: The burden of dyslipidaemia and factors associated with lipid levels among adults in rural northern Ghana: An AWI-Gen sub-study (PLoS ONE (2019) 13:11 (e0206326) 10.1371/journal.pone.0206326). PLoS One. 2019;14(2):1–21. [DOI] [PMC free article] [PubMed]
- 28.Name EA, Of N, Occupied R, Household BY, Visits I, Visit F et al. QUESTIONAIRRE FOR INDEPTH EQUITY TOOL - Male - Female.
- 29.Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. J Phys Act Heal. 2009;6(6):790–804. [DOI] [PubMed] [Google Scholar]
- 30.Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, et al. World health organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the stages of reproductive aging Workshop + 10. Menopause. 2012;19(4):387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WHO. Factors Influencing the development of overweight and obesity. Obes Prev Manag Glob epidemic. 1997;16–34.
- 33.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. [DOI] [PubMed]
- 34.Kim SH, Moon JY, Lim YM, Kim KH, Yang WI, Sung JH, et al. Association of insulin resistance and coronary artery remodeling: an intravascular ultrasound study. Cardiovasc Diabetol. 2015;14(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27(6):1487–95. [DOI] [PubMed] [Google Scholar]
- 36.Adatsi RK, Bockarie AS, Derkyi-Kwarteng L, Pappoe F, Nsiah P, Dankwa K, et al. Increased insulin resistance with reduced beta cell function in recovered COVID-19 Ghanaians. Endocr Metab Sci. 2023;13:100150. [Google Scholar]
- 37.Ingelsson E, ã„rnlã¶v J, Sundstrã¶m J, Risã©rus U, Michaã«lsson K, Byberg L. Relative importance and conjoint effects of obesity and physical inactivity for the development of insulin resistance. Eur J Prev Cardiol. 2009;16(1):28–33. [DOI] [PubMed] [Google Scholar]
- 38.Huang H, Zheng X, Wen X, Zhong J, Zhou Y, Xu L. Visceral fat correlates with insulin secretion and sensitivity independent of BMI and subcutaneous fat in Chinese with type 2 diabetes. Front Endocrinol (Lausanne). 2023;14(February):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uludağ B, Solmaz H, Alihanoğlu Yİ, Kılıç İD, Enli Y. The relationship of body mass index with insulin Resistance, hs-CRP, and Lp(a) levels in female gender. Int J Cardiovasc Acad. 2023;9(1):3–8. [Google Scholar]
- 40.Serrano Cardona L, Muñoz Mata E. Paraninfo digital. Early Hum Dev. 2013;83(1):1–11. [Google Scholar]
- 41.Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obes. 2012;19(2):81–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kodaman N, Aldrich MC, Sobota R, Asselbergs FW, Poku KA, Brown NJ, et al. Cardiovascular disease risk factors in Ghana during the rural-to-urban transition: A cross-sectional study. PLoS ONE. 2016;11(10):24–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Micah FB, Nkum BC. Lipid disorders in hospital attendants in Kumasi, Ghana. Ghana Med J. 2012;46(1):14–21. [PMC free article] [PubMed] [Google Scholar]
- 44.Kerr AG, Andersson DP, Dahlman I, Rydén M, Arner P. Adipose insulin resistance associates with dyslipidemia independent of liver resistance and involves early hormone signaling. Arterioscler Thromb Vasc Biol. 2023;43(6):1054–65. [DOI] [PubMed] [Google Scholar]
- 45.Lin D, Qi Y, Huang C, Wu M, Wang C, Li F, et al. Associations of lipid parameters with insulin resistance and diabetes: A population-based study. Clin Nutr. 2018;37(4):1423–9. [DOI] [PubMed] [Google Scholar]
- 46.Pihlajamäki J, Gylling H, Miettinen TA, Laakso M. Insulin resistance is associated with increased cholesterol synthesis and decreased cholesterol absorption in normoglycemic men. J Lipid Res. 2004;45(3):507–12. [DOI] [PubMed] [Google Scholar]
- 47.Handy RM, Holloway GP. Insights into the development of insulin resistance: unraveling the interaction of physical inactivity, lipid metabolism and mitochondrial biology. Front Physiol. 2023;14(April 2023):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santos IS, Bittencourt MS, Goulart AC, Schmidt MI, Diniz M, de Lotufo FHS. Insulin resistance is associated with carotid intima-media thickness in non-diabetic subjects. A cross-sectional analysis of the ELSA-Brasil cohort baseline. Atherosclerosis. 2017;260:34–40. [DOI] [PubMed] [Google Scholar]
- 49.Asghari G, Dehghan P, Mirmiran P, Yuzbashian E, Mahdavi M, Tohidi M, et al. Insulin metabolism markers are predictors of subclinical atherosclerosis among overweight and obese children and adolescents. BMC Pediatr. 2018;18(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: A systematic review and meta-analysis. Circulation. 2007;115(4):459–67. [DOI] [PubMed] [Google Scholar]
- 51.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Carotid-Artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. N Engl J Med. 1999;340(1):14–22. [DOI] [PubMed] [Google Scholar]
- 52.Martins FO, Conde SV. Impact of diet composition on insulin resistance. Nutrients. 2022;14(18). 10.3390/books978-3-0365-5406-8 [DOI] [PMC free article] [PubMed]
- 53.Gingras V, Rifas-Shiman SL, Taveras EM, Oken E, Hivert MF. Dietary behaviors throughout childhood are associated with adiposity and estimated insulin resistance in early adolescence: A longitudinal study. Int J Behav Nutr Phys Act. 2018;15(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ushula TW, Mamun A, Darssan D, et al. Dietary patterns and the risks of metabolic syndrome and insulin resistance among young adults: Evidence from a longitudinal study. Clinical Nutrition. 2022; 41(7):1523-1531. [DOI] [PubMed]
- 55.Lesani A, Jayedi A, Karimi M, Djafarian K, Barkhidarian B, Akbarzade Z, et al. Meal-specific dietary patterns and biomarkers of insulin resistance in a sample of Iranian adults: a cross-sectional study. Sci Rep. 2023;13(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pisprasert V, Ingram KH, Lopez-Davila MF, Munoz AJ, Garvey WT. Limitations in the use of indices using glucose and insulin levels to predict insulin sensitivity: impact of race and gender and superiority of the indices derived from oral glucose tolerance test in African Americans. Diabetes Care. 2013;36(4):845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in the supplementary files of this published article.