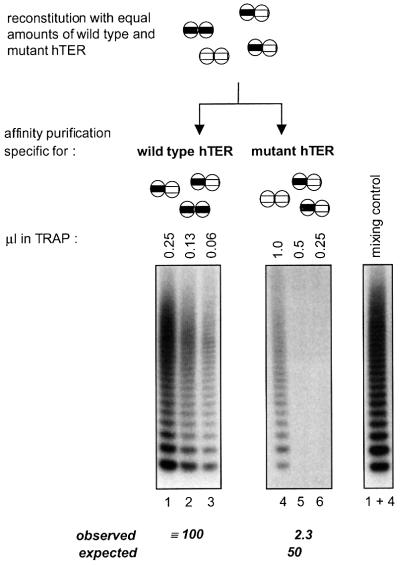
Fig. 5. Functional interaction of telomerase RNA templates. (Upper panel) Schematic outline of the distribution of homo- and heterodimers after reconstitution of telomerase with equal amounts of wild-type and mutant hTER and affinity purification. The fraction obtained with the antisense oligonucleotide specific for the mutant template lacks wild-type homodimers and vice versa. The number of wild-type templates after purification with the antisense oligonucleotide specific for the wild type is twice the number of wild-type templates present in the fraction that was purified via the mutant template. (Lower panel) The activity of eluates was measured using the TRAP assay. Below the gel, measured activities are indicated together with calculated values for non-cooperating subunits (expected), assuming that a heterodimer containing one wild-type active site would be half as active as a wild-type homodimer. The right panel shows a control experiment in which the fractions obtained with both antisense oligonucleotides were mixed after the purification (0.25 µl wild type + 1 µl mutant).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.