Abstract
Fms-like tyrosine kinase 3 (FLT3) mutation is one of the most prevalent molecular abnormalities in acute myeloid leukemia (AML), with FLT3 internal tandem duplication (FLT3-ITD) mutation being the major mutated form. AML patients with FLT3-ITD mutations have unfavorable outcomes with chemotherapy alone, while FLT3 inhibitors significantly improve patient prognoses. Recent studies have indicated that FLT3 mutated receptor has different intracellular localization of the protein, which can be specifically targeted. This review summarizes the different intracellular localization of FLT3 protein, and the influence of different positions on downstream signals. When the FLT3 receptor is located on the cell membrane, it mainly activates the downstream RAS/MAPK signaling pathway. However, the FLT3 protein located in the endoplasmic reticulum (ER) mainly activates the STAT5 signaling pathway. The wild-type FLT3 receptor is mainly located on the cell membrane, and the FLT3-ITD receptor is mainly located in the endoplasmic reticulum. We also discuss the biological mechanisms driving differential FLT3 receptors localization and potential drugs that could alter FLT3 receptors localization for therapeutic purposes. Understanding these mechanisms provides insights into FLT3 receptor biology and may guide the development of novel treatment strategies for patients with FLT3-mutated AML.
Keywords: AML, FLT3 mutation, Intracellular localization, Glycosylation, Anti-leukemia
Introduction
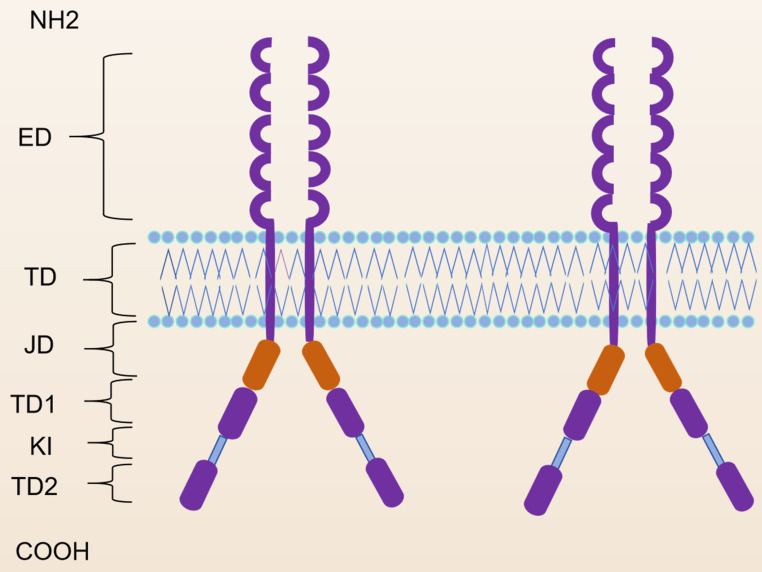
Acute myeloid leukemia (AML) is a heterogeneous malignant clonal disease originating from the transformation of immature myeloid progenitor cells in the bone marrow through recurrent genetic alterations, leading to leukemic cell proliferation and survival [1]. The cornerstone of curative treatment is intensive combination chemotherapy, typically consisting of anthracycline and cytarabine (the 3 + 7 regimen). Current standard chemotherapy can achieve cures in approximately 40%–45% of younger adults and 10%–20% of older adults with AML [2]. Certain molecular mutations play important roles in diagnosis, treatment, and prognoses of AML. FLT3 is a member of the class III receptor tyrosine kinase (RTK) family and plays a critical role in the survival, proliferation, and differentiation of hematopoietic cells [3, 4]. Structurally, FLT3 comprises five extracellular immunoglobulin-like domains, a juxtamembrane (JM) domain, a kinase insertion domain, a tyrosine kinase (TK) domain, and a C-terminal domain in the intracellular region (Fig. 1) [5]. FLT3 is expressed on normal hematopoietic stem/progenitor cells, while its ligand (FL) is produced by bone marrow stromal cells in either membrane-bound or soluble form [6, 7]. Upon binding of FL to the extracellular domain, FLT3 dimerizes, leading to transphosphorylation of tyrosine residues in the activation loop (A-loop). This activation triggers multiple intracellular signaling pathways that promote hematopoietic cell survival, proliferation, and differentiation [8]. Activating mutations in Fms-like tyrosine kinase 3 (FLT3) are among the most common genetic mutations in AML, occurring in approximately 40% of patients with cytogenetically normal AML [9, 10]. The internal tandem duplication (ITD) mutation in the JM domain of the FLT3 gene (FLT3-ITD) was first identified in AML cells in 1996 [11]. Subsequently, missense point mutations at the D835 residue and point mutations, deletions, and insertions in the codons surrounding D835 within the TK domain (FLT3-TKD) were discovered [12]. These mutations enable FLT3 to form ligand-independent dimers that undergo autophosphorylation, resulting in the constitutive activation of downstream signaling pathways and uncontrolled cell proliferation [9, 13]. FLT3-ITD, in particular, is associated with poor prognosis, including shorter event-free survival (EFS) and overall survival (OS) durations and a high relapse rate [14–16]. FLT3 inhibitors have become a cornerstone in AML treatment. In recent decades, numerous small-molecule FLT3 inhibitors have been evaluated in clinical trials for the treatment of FLT3-mutated AML. These inhibitors are categorized into first- and second-generation compounds on the basis of their kinase selectivity profiles [17]. Currently, newly emerged kinase inhibitors such as midostaurin and second-generation FLT3 inhibitors such as quizartinib are approved for first- or second-line AML treatment [18, 19], and gilteritinib can improve the prognosis of patients with relapsed/refractory FLT3-mutated AML [20]. However, leukemic cells rapidly adapt to these therapies, leading to treatment failure or relapse within weeks to months [19]. Studies have suggested that clinical resistance to FLT3 inhibitors arises from a complex interplay of genetic and epigenetic adaptations in leukemic cells, the emergence of resistant (sub)clones, and protective interactions with the bone marrow microenvironment [21, 22]. Therefore, developing novel strategies to overcome FLT3 inhibitor resistance remains a critical unmet need in AML therapy.
Fig. 1.
Molecular structure of the FLT3 protein
ED: extracellular; TD: transmembrane domain; JMD: juxtamembrane domain; TKD1: tyrosine kinase domain-1; KI: kinase insert; TKD2: tyrosine kinase domain-2.
Studies have demonstrated that the FLT3 receptor exhibits distinct intracellular localization patterns and activates different downstream signaling pathways when mutated [23–25]. Altering the intracellular localization of the FLT3 receptor can modulate tumor cell proliferation and apoptosis by activating different downstream signals [4, 26]. This review explores the differential intracellular localization of mutant and wild-type FLT3 proteins and their impact on downstream signaling pathway activation. In addition, it examines the mechanisms underlying these differences and discusses potential drugs capable of modifying FLT3 receptor localization, offering new therapeutic targets for patients with FLT3-mutated AML.
Different intracellular localizations of FLT3 receptors
Several studies have revealed that mutated transmembrane receptors exhibit distinct subcellular localization patterns within cells. Abnormal maturation and trafficking have been observed in various tyrosine kinase receptors, including the platelet-derived growth factor (PDGF), KIT, and colony-stimulating factor (CSF) 1 receptors, which, like FLT3, belong to the type III receptor tyrosine kinase (RTK) family. Western blot analysis have identified two forms of the FLT3 receptors [27–29]: a 150 kDa species representing a glycosylated complex the mature form, and a 130 kDa species representing an under-glycosylated, immature form containing mannose-rich structures. In cells expressing FLT3-ITD, the 130 kDa species predominates, whereas cells expressing wild-type FLT3 (FLT3-wt) exhibit a greater proportion of the 150 kDa species [30]. Schmidt-Arras et al. reported that in the FLT3-ITD-carrying MV4-11 cell line, the 130 kDa species was predominant, and was mainly detectable in the endoplasmic reticulum (ER) membranes, lower cell surface expression was observed in comparison with that of the wild-type receptor. Whereas in the FLT3-wt-harboring THP-1 cell line, the 150 kDa species was more abundant [27], localized mainly in the cellular surface. Other studies have also confirmed this finding. LvK et al. reported that FLT3-wt was abundantly distributed on the cell surface, while FLT3-ITD was largely localized in the ER by flow cytometry and immunofluorescence confocal microscopy [31]. Based on the studies, a conclusion can be drawn ITD mutations in FLT3 both induce ligand-independent constitutive activation of the receptor and impair trafficking, leading to prolonged residence of the receptor in the ER.
Mechanisms affecting FLT3 receptors intracellular localization
Different forms of glycosylation of the FLT3 receptors
Glycosylation is a posttranslational protein modification that plays a critical role in key biological processes, including cell growth, differentiation, and immune regulation [32]. This modification involves the covalent attachment of sugar chains to specific amino acid residues in proteins and is mediated by a series of enzymes [33, 34]. The intracellular localization of the FLT3 receptor is closely linked to its glycosylation state. FLT3 is initially synthesized as a 110 kDa protein without glycan modifications, undergoing initial glycosylation in the ER to form a 130 kDa immature protein containing oligomannose. This immature form is further processed in the Golgi apparatus to produce a 150 kDa complex-glycosylated protein, which is ultimately transported to the cell surface [27]. The 150 kDa form is more abundant in FLT3-wt cells and localized in cell surface. In FLT3-ITD cells, FLT3 receptors primarily exists in ER in the 130 kDa form, with a minor fraction in the mature form in cell surface. To further verify whether the intracellular localization of FLT3 is related to its protein glycosylation, Choudhary et al. treated the FLT3-ITD-expressing myeloid progenitor cell line 32D with the glycosylation inhibitor tunicamycin and confirmed that Flt3 surface expression was inhibited by this drug and that its expression increased in the ER [35].
Pim-1 kinase phosphorylates and stabilizes 130 kDa FLT3
The precise mechanism underlying the reduced glycosylation levels in FLT3 mutants remains unclear. Natarajan et al. [36] reported that FLT3-ITD contains a Pim-1 substrate consensus serine phosphorylation site, and they hypothesized that it might be a Pim-1 substrate. Pim-1 stabilize the FLT3-ITD protein and enable it to bind to chaperones calnexin and heat shock protein 90 (HSP-90), and mediates aberrant STAT5 signaling. On the contrary Pim-1 inhibitior decreased the expression and half-life of 130 kDa FLT3, in association with decreased FLT3-ITD binding to calnexin and HSP90, and increased 150 kDa FLT3 expression.
The presence of mutated nucleophosmin 1
Research has indicated that cytoplasmic Nucleophsmin 1 (NPM1) mutations are present in 35% of AML cases [37]. Activating mutations of FLT3 and NPM1 are often cioncidental in AML [38]. Flow cytometry analysis revealed a significant reduction of FLT3 surface expression in Flt3-D835Y+ NPM1+ bone marrow cells compared with that in Flt3−D835Y+ NPM1 wild-type control cells [39]. In addition, colocalization of FLT3-TKD with the ER marker calnexin was observed in NPM1-mutated cells but not in NPM1 wild-type cells [39]. This finding was corroborated in human AML cell lines, NPM1-mutated OCI-AML3 cells exhibited greater colocalization of FLT3 and PDI (an ER marker) than did NPM1 wild-type HL-60 cells. The study suggests the presence of NPM1 shifts FLT3-D835Y localization to the ER. Rudorf et al. generated various mutants to identify the critical domains required for the Flt3-D835Y-NPM1 interaction and demonstrated that the C-terminal region of the FLT3 kinase domain is essential for NPM1 binding. Furthermore, they demonstrated that FLT3 phosphorylation at amino acid 835 is crucial for its interaction with NPM1 [39]. In conclusion, phosphorylated FLT3-D835Y interacts with NPM1, leading to its intracellular retention and subsequent activation of STAT5 signaling [38].
Palmitoylation of FLT3-ITD
Protein subcellular localization can be regulated by lipid conjugation. Palmitoylation, a reversible lipid modification, involves the addition of a 16-carbon palmitic acid to cysteine residues of target proteins. This process is catalyzed by the ZDHHC family of palmitoyl acyltransferases (PATs) [40, 41]. Palmitoylation plays a critical role in regulating protein subcellular trafficking, stability, and enzymatic activity [42–44]. In a study by Kaosheng and colleagues [31], FLT3-ITD was found to be S-palmitoylated by the palmitoyl acyltransferase ZDHHC6. Disruption of palmitoylation redirected FLT3-ITD to the plasma membrane and altered its downstream signaling, activating AKT and extracellular signal-regulated kinase pathways in addition to STAT5. Furthermore, the inhibition of palmitoylation enhances FLT3-ITD-mediated leukemia progression in xenotransplanted mouse models [31]. The ER localization of the FLT3 receptor is linked to palmitoylation and that inhibiting palmitoylation can relocate FLT3 from the ER to the cell membrane.
In addition to the mechanisms described above, Schmidt-Arras D et al. have shown that FLT3 can be localized to the perinuclear ER through the addition of ER retention sequences containing RRR (R3) motifs [29]. These findings suggest that specific ER retention signals on the FLT3 protein may be recognized by the ER, leading to its retention, whereas wild-type FLT3 lacks or has weak retention signals, allowing it to localize primarily to the cell membrane [29]. Other studies have shown that the ER localization of the FLT3 receptor is associated with the KDEL receptor (KDELR1), a transmembrane protein involved in retrograde transport between the Golgi apparatus and the ER. KDELR1 alters the intracellular localization of the FLT3 protein by affecting its transport between the Golgi and the endoplasmic reticulum. In FLT3-ITD-expressing leukemic MV4-11 cells, downregulation of KDELR1 results in the ER expression of FLT3 and further reduces STAT5 activation, cell proliferation, and colony-forming capacities [45].
Different types of intracellular localization of FLT3 receptors activate different downstream pathways
Several studies have demonstrated that alterations in FLT3 intracellular localization significantly influence the regulation of its downstream signaling pathways (Fig. 2). Choudhary et al. reported that FLT3-ITD mostly localized to the ER activates STAT5 and upregulates its downstream target, Pim1/2, driving cell proliferation, while FLT3-ITD localized at the membrane strongly activates the MAPK/PI3K pathways with reduced STAT5 phosphorylation [35]. Köthe et al. [46] further confirmed that FLT3-ITD localized at the plasma membrane leads to constitutive activation of K-Ras. Studies have shown that altering the intracellular localization of the FLT3 receptor can change its downstream signal transduction.Kaosheng Lv et al. [31] discovered that FLT3-ITD is S-palmitoylated by the palmitoyl acyltransferase ZDHHC6. Disruption of palmitoylation redirected FLT3-ITD to the plasma membrane and activated the downstream MAPK/PI3K pathways. After addition of the tyrosine kinase inhibitor (TKI) AC220 (quizartinib), the intracellular localization of FLT3-ITD, as well as FLT3-TKD, changes to a plasma membrane localization, similar to FLT3-wt, and activates the downstream MAPK/PI3K pathways [47]. From this view, it can be seen that the cellular localization and not the mutational status is what drives differential activation signaling pathways.
Fig. 2.
Schematic representation of FLT3-ITD signaling compartmentalization
The activation of FLT3 receptors (FLT3-wt and FLT3-ITD) on the cell surface activates the MAPK and PI3K pathways. By contrast, mislocalized activation of FLT3-ITD on the ER aberrantly activates the STAT5 pathway and upregulates Pim-1/2, which in turn further phosphorylates downstream targets such as eIF4B and BAD [36].
Novel targeting therapy alters the intracellular localization of FLT3 receptors
In recent decades, numerous small-molecule FLT3 inhibitors have been evaluated in clinical trials for the treatment of FLT3-mutated AML. However, the emergence of drug resistance in FLT3-mutated leukemia has become increasingly prominent. Consequently, identifying novel therapeutic targets is critically important. Recent studies have demonstrated that various pharmacological strategies can modulate FLT3-ITD-driven oncogenic pathways by altering the intracellular localization of FLT3-ITD [31, 47, 48]. This review summarizes the current therapeutic agents that target the different cellular distributions of the FLT3 protein (Table 1).
Table 1.
Therapy targeting FLT3 intracellular localization
| Drugs | Regulation manner of FLT3 glycosylation | References |
|---|---|---|
| 2-deoxy-D-glucose | Inhibiting N-glycosylation | Larrue et al. [49] |
| Tunicamycin | Inhibiting N-glycosylation | Tsitsipatis et al. [50] |
| Statin | Inhibiting HMGCoA reductase | Williams et al. [51] |
| Pim-1 inhibitors | Inhibition of PIM phosphorylation of FLT3-ITD | Bunting KD et al. [36] |
| HSP90 inhibitors | Increase fully glycosylated form | Marzec M et al. [52] |
| Tyrosinekinase inhibitors | Increase fully glycosylated form | Reiter et al. [47] |
Glycosylation inhibitors
The FLT3 protein is localized differently within cells depending on its glycosylation status. Targeting FLT3 glycosylation can alter its localization and impact downstream signaling pathways, thereby presenting new avenues for therapeutic strategies.
2-Deoxy-D-glucose
2-Deoxy-D-glucose (2-DG), a sugar analog, is known to inhibit N-linked glycosylation synthesis and plays a significant role in suppressing glucose metabolism [53]. Larrue et al. demonstrated that 2-DG inhibits the surface expression of FLT3 in patient samples with FLT3-ITD mutations, with similar effects observed in the FLT3-wt-carrying RS4-11 cell line [49]. The inhibition of glycosylation by 2 -DG reduces cell viability and induces apoptosis in FLT3-ITD-positive leukemic cell lines and primary AML samples. One proposed mechanism is that 2-DG blocks N-linked glycosylation, leading to the accumulation of misfolded proteins and subsequent activation of ER stress (ERS), which triggers apoptosis [54, 55]. Another potential mechanism involves the inhibition of glycolysis by 2-DG. Ju et al. reported that FLT3-ITD significantly enhances aerobic glycolysis through upregulation of AKT-mediated mitochondrial hexokinase (HK2), rendering leukemia cells highly dependent on glycolysis and sensitive to glycolytic inhibition [56]. 2-DG can inhibit glycolysis, preferentially inducing severe ATP depletion and massive cell death in FLT3-ITD leukemia cells and significantly enhancing the cytotoxic effects of the FLT3 tyrosine kinase inhibitor sorafenib [56].
Tunicamycin
Tunicamycin (TM), a bacterial antibiotic, potently inhibits the transfer of activated sugars to dolichol phosphate, a critical step in N-linked protein glycosylation within the ER [57, 58]. TM effectively suppresses N-linked oligosaccharide synthesis in various cell types [59, 60]. Studies have demonstrated that low doses of TM inhibit N-glycosylation and exert antiproliferative and proapoptotic effects on FLT3-ITD-expressing human and murine cell lines by preventing FLT3-ITD glycoprotein maturation [50]. This effect is partially mediated by trapping FLT3-ITD in an underglycosylated state, thereby attenuating FLT3-ITD-driven AKT and ERK signaling. In addition, TM induces pronounced ER stress, leading to apoptosis in FLT3-ITD-positive cells through the activation of protein kinase RNA-like ER kinase (PERK) and the upregulation of CCAAT-enhancer-binding protein homologous protein (CHOP) [61]. Notably, combining TM with potent FLT3 inhibitors results in synergistic cell killing, which is highly selective for FLT3-ITD-mutated cell lines [57].
Statins
Statins function by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCoA reductase), a key enzyme in the mevalonate pathway that produces dolichol, a critical component for N-linked glycosylation [62, 63]. Several studies have demonstrated that statins suppress tumor cell proliferation both in vitro and in vivo [64–66]. For example, mevastatin has been shown to increase the sensitivity of primary AML patients to standard therapies [64], whereas lovastatin inhibits erythropoietin receptor expression through dual inhibition of glycosylation [61]. Similarly, statins inhibit the synthesis of polyterpene alcohols, thereby reducing the expression of both mature and immature FLT3 proteins in FLT3-ITD-mutated AML cells. In addition, statins diminish FLT3-ITD kinase activity by blocking receptor complex glycosylation, which alters its intracellular localization and signaling pathways, ultimately inducing apoptosis [29].
Pim-1 inhibitors
The oncogenic serine/threonine kinase Pim-1, a proviral integration site for Moloney murine leukemia virus 1, plays a critical role in regulating cytokine signaling across various cancers, including myeloma, leukemia, and prostate and breast cancers [67]. Pim-1 is known to stabilize multiple substrate proteins [68–71]. FLT3, which contains a common serine phosphorylation site recognized by Pim-1, is a direct substrate of Pim-1 [72]. Studies have shown that Pim-1 interacts directly with FLT3 and phosphorylates its serine residues, thereby stabilizing the 130 kDa FLT3 protein. This phosphorylation inhibits FLT3 glycosylation, reducing the expression of the 150 kDa FLT3 protein and promoting downstream STAT5 signaling activation. Inhibition of Pim-1 decreases the expression and half-life of the 130 kDa FLT3 protein while increasing the expression and half-life of the 150 kDa FLT3 protein [50].
HSP90 inhibitors
The increased protein synthesis rate driven by oncogenic signals often leads to protein toxicity stress, triggering the unfolded protein response (UPR) and inducing cancer cell apoptosis [73–75]. Heat shock proteins (HSPs), which function as molecular chaperones, are widely expressed in both bacteria and mammals. HSPs are classified into five major families on the basis of their molecular weight: HSP110, HSP90, HSP70, HSP60, and small heat shock proteins [52, 76, 77]. The UPR can upregulate HSP90, which interacts with numerous client proteins to maintain proteostasis, prevent degradation, and safeguard essential proteins involved in physiological processes [52], Unlike FLT3-wt, which does not bind to HSP90 even upon FL stimulation, FLT3-ITD has an abnormally long JM domain due to the insertion of repetitive sequences. HSP90 binds to FLT3-ITD, stabilizing its structure and causing its abnormal accumulation in the ER [78]. In addition, FLT3-ITD activates the UPR, further increasing HSP90 expression, which stabilizes FLT3-ITD proteins and protects AML cells from apoptosis [79, 80]. Studies have shown that HSP90 inhibitors reduce the binding of HSP90 and FLT3-ITD proteins, resulting in the decreased stability of FLT3-ITD protein and apoptosis of FLT3-ITD-positive cells. These inhibitors specifically block tyrosine phosphorylation of FLT3-ITD without affecting FL-induced phosphorylation of FLT3-wt. Consequently, downstream signaling molecules such as STAT5 are also dephosphorylated [81].
Tyrosine kinase inhibitors
TKIs are now standard of care for the treatment of patients with FLT3-mutated AML. FLT3-ITD is abnormally located in the ER as an immature protein. FLT3-ITD maturation and cell surface expression were restored by treatment with FLT3-inhibiting TKIs [82]. Reiter et al. analyzed the effects of TKIs on the localization of the FLT3 receptor and its mutants [47]. Furthermore, quizartinib increases surface expression through the upregulation of FLT3 and glycosylation of the FLT3-ITD and FLT3-D835Y mutants, increasing the drug sensitivity of FLT3-mutant cells. Lv and co-workers [31] reported that pharmacological inhibition of FLT3-ITD depalmitoylation synergized with the U.S Food and Drug Administration-approved FLT3 kinase inhibitor gilteritinib in abrogating the growth of primary FLT3-ITD AML cells.
Conclusion and future directions
This review summarizes the different intracellular localizations of the FLT3 receptor. The mechanism may be related to the glycosylation state of the FLT3 receptor, the co-expression of NPM1, and the palmitoylation of the FLT3 receptor. The different intracellular localizations of the FLT3 receptor activate different downstream signaling pathways. We further summarized the drugs that can alter the intracellular localization of FLT3 receptor, resulting in downstream signaling pathways, and can impact leukemia cell proliferation and apoptosis ultimately.This provides a new targeted treatment direction for leukemia patients with FLT3 mutations.
Acknowledgements
We would like to thank the staff of the Department of Hematology at the First Hospital of Jilin University for their assistance with this review. We thank LetPub (www.letpub.com.cn) for its linguistic assistance during the preparation of this manuscript.
Author contributions
S.F. wrote the manuscript. S.F., S.L., and G.S.J. collected, analyzed, and summarized the data. S.F., S.L., and G.S.J. conceptualized this review. S.L. and G.S.J. revised the review. The final manuscript was read and approved by all the authors.
Funding sources
This study was funded by the Department of Science and Technology of Jilin Province (YDZJ202201ZYTS606) and the Natural Science Foundation of China (82370153).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Long Su, Email: sulong@jlu.edu.cn.
Sujun Gao, Email: sjgao@jlu.edu.cn.
References
- 1.Hospital M-A, Green AS, Maciel TT, Moura IC, Leung AY, Bouscary D, Tamburini J (2017) FLT3 inhibitors: clinical potential in acute myeloid leukemia. OncoTargets Ther 10:607–615. 10.2147/ott.S103790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yamawaki K, Shiina I, Murata T, Tateyama S, Maekawa Y, Niwa M, Shimonaka M, Okamoto K, Suzuki T, Nishida T, Abe R, Obata Y (2021) FLT3-ITD transduces autonomous growth signals during its biosynthetic trafficking in acute myelogenous leukemia cells. Sci Rep. 10.1038/s41598-021-02221-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunting KD, Volpe G, Clarke M, Garcìa P, Walton DS, Vegiopoulos A, Del Pozzo W, O’Neill LP, Frampton J, Dumon S (2015) Regulation of the Flt3 gene in Haematopoietic stem and early progenitor cells. PLoS ONE 10(9). 10.1371/journal.pone.0138257 [DOI] [PMC free article] [PubMed]
- 4.Hu X, Chen F (2019) Targeting on glycosylation of mutant FLT3 in acute myeloid leukemia. Hematology 24(1):651–660. 10.1080/16078454.2019.1666219 [DOI] [PubMed] [Google Scholar]
- 5.Griffith J, Black J, Faerman C, Swenson L, Wynn M, Lu F, Lippke J, Saxena K (2004) The structural basis for autoinhibition of FLT3 by the juxtamembrane domain. Mol Cell 13(2):169–178. 10.1016/s1097-2765(03)00505-7 [DOI] [PubMed] [Google Scholar]
- 6.Small D, Levenstein M, Kim E, Carow C, Amin S, Rockwell P, Witte L, Burrow C, Ratajczak MZ, Gewirtz AM (1994) STK-1, the human homolog of Flk-2/Flt-3, is selectively expressed in CD34 + human bone marrow cells and is involved in the proliferation of early progenitor/stem cells. Proc Natl Acad Sci U S A 91(2):459–463. 10.1073/pnas.91.2.459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannum C, Culpepper J, Campbell D, McClanahan T, Zurawski S, Kastelein R, Bazan JF, Hudak S, Wagner J, Mattson J, Luh J, Duda G, Martina N, Peterson D, Menon S, Shanafelt A, Muench M, Kelner G, Namikawa R, Rennick D, Roncarolo MG, Zlotnik A, Rosnet O, Dubreuil P, Birnbaum D, Lee F (1994) Ligand for FLT3/FLK2 receptor tyrosine kinase regulates growth of haematopoietic stem cells and is encoded by variant RNAs. Nature 368(6472):643–648. 10.1038/368643a0 [DOI] [PubMed] [Google Scholar]
- 8.Weiss A, Schlessinger J (1998) Switching signals on or off by receptor dimerization. Cell 94(3):277–280. 10.1016/s0092-8674(00)81469-5 [DOI] [PubMed] [Google Scholar]
- 9.Gilliland DG, Griffin JD (2002) The roles of FLT3 in hematopoiesis and leukemia. Blood 100(5):1532–1542. 10.1182/blood-2002-02-0492 [DOI] [PubMed] [Google Scholar]
- 10.Stirewalt DL, Radich JP (2003) The role of FLT3 in haematopoietic malignancies. Nat Rev Cancer 3(9):650–665. 10.1038/nrc1169 [DOI] [PubMed] [Google Scholar]
- 11.Kiyoi H, Kawashima N, Ishikawa Y (2019) FLT3 mutations in acute myeloid leukemia: therapeutic paradigm beyond inhibitor development. Cancer Sci 111(2):312–322. 10.1111/cas.14274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamamoto Y (2001) Activating mutation of D835 within the activation loop of FLT3 in human hematologic malignancies. Blood 97(8):2434–2439. 10.1182/blood.V97.8.2434 [DOI] [PubMed] [Google Scholar]
- 13.Markovic A, MacKenzie KL, Lock RB (2005) FLT-3: a new focus in the understanding of acute leukemia. Int J Biochem Cell Biol 37(6):1168–1172. 10.1016/j.biocel.2004.12.005 [DOI] [PubMed] [Google Scholar]
- 14.Levis M, Small D (2003) FLT3: itdoes matter in leukemia. Leukemia 17(9):1738–1752. 10.1038/sj.leu.2403099 [DOI] [PubMed] [Google Scholar]
- 15.Mrózek K, Marcucci G, Paschka P, Whitman SP, Bloomfield CD (2006) Clinical relevance of mutations and gene-expression changes in adult acute myeloid leukemia with normal cytogenetics: are we ready for a prognostically prioritized molecular classification? Blood 109(2):431–448. 10.1182/blood-2006-06-001149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergeron J, Capo-Chichi J-M, Tsui H, Mahe E, Berardi P, Minden MD, Brandwein JM, Schuh AC (2023) The clinical utility of FLT3 mutation testing in acute leukemia: a Canadian consensus. Curr Oncol 30(12):10410–10436. 10.3390/curroncol30120759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ke Y-Y, Singh VK, Coumar MS, Hsu YC, Wang W-C, Song J-S, Chen C-H, Lin W-H, Wu S-H, Hsu JTA, Shih C, Hsieh H-P (2015) Homology modeling of DFG-in FMS-like tyrosine kinase 3 (FLT3) and structure-based virtual screening for inhibitor identification. Sci Rep. 10.1038/srep11702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, Thiede C, Prior TW, Döhner K, Marcucci G, Lo-Coco F, Klisovic RB, Wei A, Sierra J, Sanz MA, Brandwein JM, de Witte T, Niederwieser D, Appelbaum FR, Medeiros BC, Tallman MS, Krauter J, Schlenk RF, Ganser A, Serve H, Ehninger G, Amadori S, Larson RA, Döhner H (2017) Midostaurin plus chemotherapy for acute myeloid leukemia with aFLT3Mutation. N Engl J Med 377(5):454–464. 10.1056/NEJMoa1614359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortes JE, Tallman MS, Schiller GJ, Trone D, Gammon G, Goldberg SL, Perl AE, Marie J-P, Martinelli G, Kantarjian HM, Levis MJ (2018) Phase 2b study of 2 dosing regimens of Quizartinib monotherapy in FLT3-ITD–mutated, relapsed or refractory AML. Blood 132(6):598–607. 10.1182/blood-2018-01-821629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perl AE, Larson RA, Podoltsev NA, Strickland S, Wang ES, Atallah E, Schiller GJ, Martinelli G, Neubauer A, Sierra J, Montesinos P, Récher C, Yoon S-S, Hosono N, Onozawa M, Chiba S, Kim H-J, Hasabou N, Lu Q, Tiu R, Levis MJ (2022) Follow-up of patients with R/R FLT3-mutation–positive AML treated with gilteritinib in the phase 3 ADMIRAL trial. Blood 139(23):3366–3375. 10.1182/blood.2021011583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weisberg E, Barrett R, Liu Q, Stone R, Gray N, Griffin JD (2009) FLT3 inhibition and mechanisms of drug resistance in mutant FLT3-positive AML. Drug Resist Updat 12(3):81–89. 10.1016/j.drup.2009.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholl S, Fleischmann M, Schnetzke U, Heidel FH (2020) Molecular mechanisms of resistance to FLT3 inhibitors in acute myeloid leukemia: ongoing challenges and future treatments. Cells. 10.3390/cells9112493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mizuki M, Fenski R, Halfter H, Matsumura I, Schmidt R, Müller C, Grüning W, Kratz-Albers K, Serve S, Steur C, Büchner T, Kienast J, Kanakura Y, Berdel WE, Serve H (2000) Flt3 mutations from patients with acute myeloid leukemia induce transformation of 32D cells mediated by the Ras and STAT5 pathways. Blood 96(12):3907–3914. 10.1182/blood.V96.12.3907 [PubMed] [Google Scholar]
- 24.Vempati S, Reindl C, Wolf U, Kern R, Petropoulos K, Naidu VM, Buske C, Hiddemann W, Kohl TM, Spiekermann K (2008) Transformation by oncogenic mutants and ligand-dependent activation of FLT3 wild-type requires the tyrosine residues 589 and 591. Clin Cancer Res 14(14):4437–4445. 10.1158/1078-0432.Ccr-07-1873 [DOI] [PubMed] [Google Scholar]
- 25.Choudhary C, Schwäble J, Brandts C, Tickenbrock L, Sargin Bl, Kindler T, Fischer T, Berdel WE, Müller-Tidow C, Serve H (2005) AML-associated Flt3 kinase domain mutations show signal transduction differences compared with Flt3 ITD mutations. Blood 106(1):265–273. 10.1182/blood-2004-07-2942 [DOI] [PubMed] [Google Scholar]
- 26.Moloney JN, Stanicka J, Cotter TG (2017) Subcellular localization of the FLT3-ITD oncogene plays a significant role in the production of NOX- and p22phox-derived reactive oxygen species in acute myeloid leukemia. Leuk Res 52:34–42. 10.1016/j.leukres.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Arras D-E, Böhmer A, Markova B, Choudhary C, Serve H, Böhmer F-D (2023) Tyrosine phosphorylation regulates maturation of receptor tyrosine kinases. Mol Cell Biol 25(9):3690–3703. 10.1128/mcb.25.9.3690-3703.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koch S, Jacobi A, Ryser M, Ehninger G, Thiede C (2008) Abnormal localization and accumulation of FLT3-ITD, a mutant receptor tyrosine kinase involved in leukemogenesis. Cells Tissues Organs 188(1–2):225–235. 10.1159/000118788 [DOI] [PubMed] [Google Scholar]
- 29.Schmidt-Arras D, Böhmer S-A, Koch S, Müller JP, Blei L, Cornils H, Bauer R, Korasikha S, Thiede C, Böhmer F-D (2009) Anchoring of FLT3 in the endoplasmic reticulum alters signaling quality. Blood 113(15):3568–3576. 10.1182/blood-2007-10-121426 [DOI] [PubMed] [Google Scholar]
- 30.Zhang S, Broxmeyer HE (1999) P85 subunit of PI3 kinase does not bind to human Flt3 receptor, but associates with SHP2, SHIP, and a tyrosine-phosphorylated 100-kDa protein in Flt3 ligand-stimulated hematopoietic cells. Biochem Biophys Res Commun 254(2):440–445. 10.1006/bbrc.1998.9959 [DOI] [PubMed] [Google Scholar]
- 31.Lv K, Ren J-G, Han X, Gui J, Gong C, Tong W (2021) Depalmitoylation rewires FLT3-ITD signaling and exacerbates leukemia progression. Blood 138(22):2244–2255. 10.1182/blood.2021011582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vasconcelos-dos-Santos A, Oliveira IA, Lucena MC, Mantuano NR, Whelan SA, Dias WB, Todeschini AR (2015) Biosynthetic machinery involved in aberrant glycosylation: promising targets for developing of drugs against cancer. Front Oncol 5. 10.3389/fonc.2015.00138 [DOI] [PMC free article] [PubMed]
- 33.Mori S, Aoyagi Y, Yanagi M, Suzuki Y, Asakura H (2008) Serum N-acetylglucosaminyltransferase III activities in hepatocellular carcinoma. J Gastroenterol Hepatol 13(6):610–619. 10.1111/j.1440-1746.1998.tb00699.x [DOI] [PubMed] [Google Scholar]
- 34.Dube DH, Bertozzi CR (2005) Glycans in cancer and inflammation — potential for therapeutics and diagnostics. Nat Rev Drug Discov 4(6):477–488. 10.1038/nrd1751 [DOI] [PubMed] [Google Scholar]
- 35.Choudhary C, Olsen JV, Brandts C, Cox J, Reddy PNG, Böhmer FD, Gerke V, Schmidt-Arras D-E, Berdel WE, Müller-Tidow C, Mann M, Serve H (2009) Mislocalized activation of oncogenic RTKs switches downstream signaling outcomes. Mol Cell 36(2):326–339. 10.1016/j.molcel.2009.09.019 [DOI] [PubMed] [Google Scholar]
- 36.Bunting KD, Natarajan K, Xie Y, Burcu M, Linn DE, Qiu Y, Baer MR (2013) Pim-1 Kinase Phosphorylates and Stabilizes 130 kDa FLT3 and Promotes Aberrant STAT5 Signaling in Acute Myeloid Leukemia with FLT3 Internal Tandem Duplication. PLoS ONE 8(9). 10.1371/journal.pone.0074653 [DOI] [PMC free article] [PubMed]
- 37.Hindley A, Catherwood MA, McMullin MF, Mills KI (2021) Significance of NPM1 gene mutations in AML. Int J Mol Sci. 10.3390/ijms221810040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boddu P, Kantarjian H, Borthakur G, Kadia T, Daver N, Pierce S, Andreeff M, Ravandi F, Cortes J, Kornblau SM (2017) Co-occurrence of FLT3-TKD and NPM1 mutations defines a highly favorable prognostic AML group. Blood Adv 1(19):1546–1550. 10.1182/bloodadvances.2017009019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rudorf A, Müller TA, Klingeberg C, Kreutmair S, Poggio T, Gorantla SP, Rückert T, Schmitt-Graeff A, Gengenbacher A, Paschka P, Baldus C, Zeiser R, Vassiliou GS, Bradley A, Duyster J, Illert AL (2019) NPM1c alters FLT3-D835Y localization and signaling in acute myeloid leukemia. Blood 134(4):383–388. 10.1182/blood.2018883140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aicart-Ramos C, Valero RA, Rodriguez-Crespo I (2011) Protein palmitoylation and subcellular trafficking. Biochimica et biophysica acta (BBA) - Biomembranes 1808:122981–2994. 10.1016/j.bbamem.2011.07.009 [DOI] [PubMed] [Google Scholar]
- 41.Fernández-Hernando C, Fukata M, Bernatchez PN, Fukata Y, Lin MI, Bredt DS, Sessa WC (2006) Identification of Golgi-localized acyl transferases that palmitoylate and regulate endothelial nitric oxide synthase. J Cell Biol 174(3):369–377. 10.1083/jcb.200601051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Runkle Kristin B, Kharbanda A, Stypulkowski E, Cao X-J, Wang W, Garcia Benjamin A, Witze Eric S (2016) Inhibition of DHHC20-mediated EGFR palmitoylation creates a dependence on EGFR signaling. Mol Cell 62(3):385–396. 10.1016/j.molcel.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kharbanda A, Walter DM, Gudiel AA, Schek N, Feldser DM, Witze ES (2020) Blocking EGFR palmitoylation suppresses PI3K signaling and mutant KRAS lung tumorigenesis. Sci Signal. 10.1126/scisignal.aax2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kadry YA, Lee J-Y, Witze ES (2021) Regulation of EGFR signalling by palmitoylation and its role in tumorigenesis. Open Biol. 10.1098/rsob.210033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caldarelli A, Müller JP, Paskowski-Rogacz M, Herrmann K, Bauer R, Koch S, Heninger AK, Krastev D, Ding L, Kasper S, Fischer T, Brodhun M, Böhmer FD, Buchholz F (2013) A genome-wide RNAi screen identifies proteins modulating aberrant FLT3-ITD signaling. Leukemia 27(12):2301–2310. 10.1038/leu.2013.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Köthe S, Müller JP, Böhmer S-A, Tschongov T, Fricke M, Koch S, Thiede C, Requardt RP, Rubio I, Böhmer FD (2013) Features of Ras activation by a mislocalized oncogenic tyrosine kinase: FLT3 ITD signals via K-Ras at the plasma membrane of acute myeloid leukemia cells. J Cell Sci 4746–4755. 10.1242/jcs.131789 [DOI] [PubMed]
- 47.Reiter K, Polzer H, Krupka C, Maiser A, Vick B, Rothenberg-Thurley M, Metzeler KH, Dörfel D, Salih HR, Jung G, Nößner E, Jeremias I, Hiddemann W, Leonhardt H, Spiekermann K, Subklewe M, Greif PA (2017) Tyrosine kinase inhibition increases the cell surface localization of FLT3-ITD and enhances FLT3-directed immunotherapy of acute myeloid leukemia. Leukemia 32(2):313–322. 10.1038/leu.2017.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fleischmann M, Fischer M, Schnetzke U, Fortner C, Kirkpatrick J, Heidel FH, Hochhaus A, Scholl S (2021) Modulation of FLT3-ITD localization and targeting of distinct downstream signaling pathways as potential strategies to overcome FLT3-inhibitor resistance. Cells. 10.3390/cells10112992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Larrue C, Saland E, Vergez F, Serhan N, Delabesse E, Mansat-De Mas V, Hospital M-A, Tamburini J, Manenti S, Sarry JE, Récher C (2015) Antileukemic activity of 2-Deoxy-d-glucose through inhibition of N-linked glycosylation in acute myeloid leukemia with FLT3-ITD or c-KIT mutations. Mol Cancer Ther 14(10):2364–2373. 10.1158/1535-7163.Mct-15-0163 [DOI] [PubMed] [Google Scholar]
- 50.Tsitsipatis D, Jayavelu AK, Müller JP, Bauer R, Schmidt-Arras D, Mahboobi S, Schnöder TM, Heidel F, Böhmer F-D (2017) Synergistic killing of FLT3ITD-positive AML cells by combined inhibition of tyrosine-kinase activity and N-glycosylation. Oncotarget 8(16):26613–26624. 10.18632/oncotarget.15772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Williams AB, Li L, Nguyen B, Brown P, Levis M, Small D (2012) Fluvastatin inhibits FLT3 glycosylation in human and murine cells and prolongs survival of mice with FLT3/ITD leukemia. Blood 120(15):3069–3079. 10.1182/blood-2012-01-403493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marzec M, Eletto D, Argon Y (2012) GRP94: an HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochimica et Biophysica Acta (BBA) 1823(3):774–787. 10.1016/j.bbamcr.2011.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dills WL, Kwong E, Covey TR, Nesheim MC (1984) Effects of diets deficient in glucose and glucose precursors on the growth of the walker carcinosarcoma 256 in rats. J Nutr 114(11):2097–2106. 10.1093/jn/114.11.2097 [DOI] [PubMed] [Google Scholar]
- 54.Xi H, Kurtoglu M, Liu H, Wangpaichitr M, You M, Liu X, Savaraj N, Lampidis TJ (2010) 2-deoxy-d-glucose activates autophagy via Endoplasmic reticulum stress rather than ATP depletion. Cancer Chemother Pharmacol 67(4):899–910. 10.1007/s00280-010-1391-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kurtoglu M, Gao N, Shang J, Maher JC, Lehrman MA, Wangpaichitr M, Savaraj N, Lane AN, Lampidis TJ (2007) Under normoxia, 2-deoxy-d-glucose elicits cell death in select tumor types not by inhibition of glycolysis but by interfering with N-linked glycosylation. Mol Cancer Ther 6(11):3049–3058. 10.1158/1535-7163.Mct-07-0310 [DOI] [PubMed] [Google Scholar]
- 56.Ju HQ, Zhan G, Huang A, Sun Y, Wen S, Yang J, Lu Wh, Xu Rh, Li J, Li Y, Garcia-Manero G, Huang P, Hu Y (2017) ITD mutation in FLT3 tyrosine kinase promotes Warburg effect and renders therapeutic sensitivity to glycolytic inhibition. Leukemia 31(10):2143–2150. 10.1038/leu.2017.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Keenan RW, Hamill RL, Occolowitz JL, Elbein AD (2002) Biological activities of isolated tunicamycin and streptovirudin fractions. Biochemistry 20(10):2968–2973. 10.1021/bi00513a039 [DOI] [PubMed] [Google Scholar]
- 58.Yoon D, Moon JH, Cho A, Boo H, Cha JS, Lee Y, Yoo J (2023) Structure-based insight on the mechanism of N-glycosylation inhibition by tunicamycin. Mol Cells 46(6):337–344. 10.14348/molcells.2023.0001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Han X, Zhang X, Li H, Huang S, Zhang S, Wang F, Shi Y (2015) Tunicamycin enhances the antitumor activity of trastuzumab on breast cancer in vitro and in vivo. Oncotarget 6(36):38912–38925. 10.18632/oncotarget.5334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang S, Wang D, Zhang S, Huang X, Wang D, Ijaz M, Shi Y (2017) Tunicamycin potentiates paclitaxel-induced apoptosis through inhibition of PI3K/AKT and MAPK pathways in breast cancer. Cancer Chemother Pharmacol 80(4):685–696. 10.1007/s00280-017-3393-7 [DOI] [PubMed] [Google Scholar]
- 61.Hamadmad SN, Hohl RJ (2007) Lovastatin suppresses erythropoietin receptor surface expression through dual inhibition of glycosylation and geranylgeranylation. Biochem Pharmacol 74(4):590–600. 10.1016/j.bcp.2007.04.028 [DOI] [PubMed] [Google Scholar]
- 62.Corsini A (1995) Pharmacology of competitive inhibitors of HMg-CoA reductase. Pharmacol Res 31(1):9–27. 10.1016/1043-6618(95)80042-5 [DOI] [PubMed] [Google Scholar]
- 63.Mo H, Elson CE (2016) Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Exp Biol Med (Maywood) 229(7):567–585. 10.1177/153537020422900701 [DOI] [PubMed] [Google Scholar]
- 64.Stirewalt DL, Appelbaum FR, Willman CL, Zager RA, Banker DE (2003) Mevastatin can increase toxicity in primary AMLs exposed to standard therapeutic agents, but statin efficacy is not simply associated with Ras hotspot mutations or overexpression. Leuk Res 27(2):133–145. 10.1016/s0145-2126(02)00085-1 [DOI] [PubMed] [Google Scholar]
- 65.Martirosyan A, Clendening JW, Goard CA, Penn LZ (2010) Lovastatin induces apoptosis of ovarian cancer cells and synergizes with doxorubicin: potential therapeutic relevance. BMC Cancer 10(1):103. 10.1186/1471-2407-10-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Holstein SA, Knapp HR, Clamon GH, Murry DJ, Hohl RJ (2005) Pharmacodynamic effects of high dose lovastatin in subjects with advanced malignancies. Cancer Chemother Pharmacol 57(2):155–164. 10.1007/s00280-005-0013-8 [DOI] [PubMed] [Google Scholar]
- 67.Tahvanainen J, Kyläniemi MK, Kanduri K, Gupta B, Lähteenmäki H, Kallonen T, Rajavuori A, Rasool O, Koskinen PJ, Rao KVS, Lähdesmäki H, Lahesmaa R (2013) Proviral integration site for Moloney murine leukemia virus (PIM) kinases promote human T helper 1 cell differentiation. J Biol Chem 288(5):3048–3058. 10.1074/jbc.M112.361709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y, Wang Z, Magnuson NS (2007) Pim-1 kinase-dependent phosphorylation of p21Cip1/WAF1 regulates its stability and cellular localization in H1299 cells. Mol Cancer Res 5(9):909–922. 10.1158/1541-7786.Mcr-06-0388 [DOI] [PubMed] [Google Scholar]
- 69.Chen XP, Losman JA, Cowan S, Donahue E, Fay S, Vuong BQ, Nawijn MC, Capece D, Cohan VL, Rothman P (2002) Pim serine/threonine kinases regulate the stability of Socs-1 protein. Proc Natl Acad Sci U S A 99(4):2175–2180. 10.1073/pnas.042035699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim HR, Oh BC, Choi JK, Bae SC (2008) Pim-1 kinase phosphorylates and stabilizes RUNX3 and alters its subcellular localization. J Cell Biochem 105(4):1048–1058. 10.1002/jcb.21906 [DOI] [PubMed] [Google Scholar]
- 71.Zhang Y, Wang Z, Li X, Magnuson NS (2008) Pim kinase-dependent inhibition of c-Myc degradation. Oncogene 27(35):4809–4819. 10.1038/onc.2008.123 [DOI] [PubMed] [Google Scholar]
- 72.Fathi AT, Arowojolu O, Swinnen I, Sato T, Rajkhowa T, Small D, Marmsater F, Robinson JE, Gross SD, Martinson M, Allen S, Kallan NC, Levis M (2012) A potential therapeutic target for FLT3-ITD AML: PIM1 kinase. Leuk Res 36(2):224–231. 10.1016/j.leukres.2011.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hazari YM, Bashir A, Haq Eu, Fazili KM (2016) Emerging tale of UPR and cancer: an essentiality for malignancy. Tumor Biol 37(11):14381–14390. 10.1007/s13277-016-5343-0 [DOI] [PubMed] [Google Scholar]
- 74.Papaioannou A, Chevet E (2017) Driving Cancer Tumorigenesis and Metastasis Through UPR Signaling. In: Coordinating Organismal Physiology Through the Unfolded Protein Response. Current Topics in Microbiology and Immunology. pp 159–192. 10.1007/82_2017_36 [DOI] [PubMed]
- 75.Madden E, Logue SE, Healy SJ, Manie S, Samali A (2018) The role of the unfolded protein response in cancer progression: from oncogenesis to chemoresistance. Biol Cell 111(1):1–17. 10.1111/boc.201800050 [DOI] [PubMed] [Google Scholar]
- 76.Hoter A, El-Sabban ME, Naim HY (2018) The HSP90 family: structure, regulation, function, and implications in health and disease. Int J Mol Sci. 10.3390/ijms19092560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Albakova Z (2024) HSP90 multi-functionality in cancer. Front Immunol. 10.3389/fimmu.2024.1436973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rutherford SL, Lindquist S (1998) Hsp90 as a capacitor for morphological evolution. Nature 396(6709):336–342. 10.1038/24550 [DOI] [PubMed] [Google Scholar]
- 79.Han S-Y (2022) Small molecule induced FLT3 degradation. Pharmaceuticals 15(3):320. 10.3390/ph15030320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ly BTK, Chi HT (2018) ETV6/FLT3 fusion is a novel client protein of Hsp90. Oncol Res Featuring Preclinical Clin Cancer Ther 26(8):1201–1205. 10.3727/096504018x15154104709325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Minami Y, Kiyoi H, Yamamoto Y, Yamamoto K, Ueda R, Saito H, Naoe T (2002) Selective apoptosis of tandemly duplicated FLT3-transformed leukemia cells by Hsp90 inhibitors. Leukemia 16(8):1535–1540. 10.1038/sj.leu.2402558 [DOI] [PubMed] [Google Scholar]
- 82.Weisberg E, Boulton C, Kelly LM, Manley P, Fabbro D, Meyer T, Gilliland DG, Griffin JD (2002) Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitor PKC412. Cancer Cell 1(5):433–443. 10.1016/s1535-6108(02)00069-7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.