Abstract
This study aimed to characterize transcriptomic alterations and the effects of demographic and behavioral risk factors on disease-associated gene expression patterns in late-stage keratoconus (KC). Corneal stromal tissues from 31 KC patients and four normal donors underwent bulk RNA sequencing. Differentially expressed genes (DEGs) and weighted gene co-expression network analysis (WGCNA) were used to identify key molecular changes. Subgroup analyses based on sex, allergy status, eye rubbing intensity (ERI), body mass index (BMI), and childhood socioeconomic status (SES) were conducted using gene set enrichment analysis (GSEA) and WGCNA. Key genes from WGCNA modules were validated by quantitative real-time PCR (qRT-PCR) and three public transcriptomic datasets. Enrichment analyses based on the 4469 identified DEGs (Padj < 0.05) revealed suppression of extracellular matrix (ECM) organization, axon guidance, synaptic signaling, and immune activity (Padj < 0.05). Subgroup GSEA demonstrated that high ERI correlated with ECM pathways, elevated BMI correlated with mitochondrial dysfunction, and low childhood SES correlated with upregulated mitochondrial metabolism and immune activation (Padj < 0.05). Chromatin regulation and antioxidant activity were enriched in females (Padj < 0.05). No transcriptomic associations were detected for allergy status. WGCNA revealed ERI-related modules involving Wnt and PI3K-Akt signaling and SES-related modules implicating cholinergic synapse and axon guidance pathways (Padj < 0.05). Validation using qRT-PCR and public datasets confirmed the downregulation of all 15 selected genes in KC (Padj < 0.05). qRT-PCR also confirmed differential expression of five subgroup-associated genes across KC subgroups (P < 0.05). These findings emphasize the neural–immune–stromal axis dysregulation in KC and elucidate the contribution of common risk factors to disease progression.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-21254-5.
Keywords: Transcriptome, Keratoconus, Gene regulation, Risk factors
Subject terms: Biomarkers, Computational biology and bioinformatics, Diseases, Genetics, Immunology, Medical research, Molecular biology
Introduction
Keratoconus (KC) is a progressive corneal ectasia characterized by central or paracentral stromal thinning and anterior protrusion, leading to progressive myopia, irregular astigmatism, and significant visual impairment1. The complex etiology of KC is multifactorial, involving genetic predisposition, metabolic dysfunction, and environmental exposures2,3. KC arises from environmental or behavioral insults superimposed on a background of genetic susceptibility4. The risk factors for KC include eye rubbing5, atopy6, sex7, body mass index (BMI)7, and socioeconomic status (SES)8.
Recent advances in high-throughput omics technologies contributed to a deeper understanding of the molecular landscape of KC8, and transcriptomic profiling of different corneal layers clarified the pathological process for the onset of KC. Epithelial stress triggered by mechanical or inflammatory stimuli (e.g., eye rubbing or allergens) activates innate immune responses, inflammation, acute phase responses, and oxidative stress responses10,11. These epithelial factors alter the function and viability of the underlying stromal keratocytes4, leading to impaired extracellular matrix (ECM) synthesis and remodeling. The altered ECM compromises corneal biomechanics and establishes a feed-forward loop of tissue degradation12,13.
Despite advances in modeling the early pathogenesis of KC, the transcriptomic landscape and molecular alterations underlying disease progression remain unclear. Previous transcriptomic studies involving stromal samples have identified responses to mechanical stress14, as well as inflammatory and immune-related changes15, including altered immune cell infiltration16. Another study suggested that stromal cell loss driven by antiproliferative and hyperapoptotic phenotypes may contribute to KC pathogenesis17. However, these findings provide only a fragmented view, and a comprehensive understanding of the molecular transitions involved in KC progression is still lacking. Furthermore, the impact of clinical and demographic factors on these molecular changes has not been systematically investigated13.
To address these gaps, this study aimed to characterize transcriptomic alterations in late-stage KC using comparative analyses of stromal tissues from patients with KC and donor corneas. The associations of transcriptomic profiles with multidimensional risk factors were assessed to elucidate the molecular mechanisms underlying KC progression and the interplay of KC progression with multifactorial risks18.
Methods
Study design and participants
This prospective study included patients who underwent deep anterior lamellar keratoplasty for KC at the Centre for Sight, Peking University Third Hospital in Beijing, China, between January 1, 2024, and December 1, 2024. Human donor corneas were procured from the Peking University Third Hospital Eye Bank in Beijing, China, as controls. Written informed consent was obtained from all patients or the legal guardians of individuals under 18 years and the control donors’ next-of-kin for research purposes. The study protocol was approved by the Medical Science Research Ethics Committee of Peking University Third Hospital (Protocol No. M2023859) and adhered strictly to the principles of the Declaration of Helsinki.
Inclusion and exclusion criteria
The inclusion criteria for the KC group were as follows: diagnosed with primary KC without corneal hydrops or scarring, no history of ocular surgery, and no preoperative ocular complications, including corneal ulcers, keratitis, eye trauma, or cataracts. Patients with severe systemic diseases or genetic disorders were excluded from the study. No other complications or contraindications, including previous eye diseases, ocular surgery, chronic systemic autoimmune or inflammatory conditions, or transmissible diseases, were observed in donor corneas. Slit-lamp examination confirmed the absence of infection, corneal neovascularization, abnormal corneal morphology, pterygium, synechiae, or signs of tumors or metastases in patients with KC19.
Clinical data
All patients with KC underwent preoperative visual acuity examinations, slit lamp examinations, fundoscopy, intraocular pressure measurements, autorefractions (Topcon RM 8800, Topcon Corporation, Tokyo, Japan), phoropters (Topcon CV-5000, Topcon Corporation, Tokyo, Japan), trial lenses, anterior segment optical coherence tomography (Visante, Zeiss, Oberkochen, Germany), and corneal tomography (Pentacam, Oculus, Washington, DC, USA). All patients exhibited contact lens intolerance and did not wear contact lenses for at least 6 months before the study. Height and weight were measured using a wall-mounted stadiometer and an electronic scale, respectively, while the participants were wearing light clothing and no shoes. BMIs were calculated using the standard formula (weight [kg]/height [m]2).
A questionnaire survey was administered by trained staff through face-to-face interviews during the patients’ initial hospital visits. Data on sex, eye rubbing intensity (ERI), parental educational attainment and occupational status, allergy and allergic disease history, family history of KC, and any history of ocular or systemic diseases were collected. The patients with KC were stratified into subgroups based on sex (male vs. female), ERI (high vs. low), BMI (high vs. low), childhood socioeconomic status (SES; low vs. high), and allergy status (yes vs. no). Eye rubbing frequency was rated as Never/Rarely, Occasionally, Sometimes, Frequently, or Always; intensity was rated as Light, Medium, or Heavy. Participants were classified as having high ERI if they reported either a frequency of “Frequently” or “Always,” or an intensity of “Heavy.” All others were classified as ERI-low. Following Chinese guidelines20, patients were categorized into normal/underweight and overweight/obese BMI subgroups (< 24 vs. ≥24 kg/m², respectively). Childhood SES was assessed using the Hollingshead Four-Factor Index of Social Status, which integrates parental education and occupation into a composite score; the higher parental score was used for two-parent households21. SES scores were categorized into high SES (upper, upper-middle, and middle class: 30–66) and low SES (working and lower class: 8–29) subgroups. Allergy status was defined as a history of allergy or allergic diseases.
RNA extraction
Following corneal button excision, the corneal stroma was immediately immersed in an RNA stabilization solution (RNAlater, Qiagen, Hilden, Germany) and stored at − 80 °C until RNA extraction. Human donor corneas were obtained and preserved in Optisol-GS storage medium (Bausch & Lomb, Irvine, CA, USA) at 4 °C within 24 h postmortem. RNA extraction was performed within 14 days of tissue preservation. Approximately 100 mg of tissue was transferred into liquid nitrogen and ground with a pre-cooled mortar and pestle. Total RNA was extracted and purified using the RNAprep Pure Tissue Kit (DP431, TIANGEN, Beijing, China), following the manufacturer’s instructions. The quality and quantity of purified RNA were assessed using the RNA Nano 6000 Assay Kit on a Bioanalyzer 2100 system (Agilent Technologies, Santa Clara, CA, USA). RNA pellets were resuspended in 20 µL RNase-free H₂O, and the total RNA samples were stored at − 80 °C.
Total RNA library preparation and sequencing
Sequencing libraries were generated using a NEBNext UltraTM RNA Library Prep Kit for Illumina (NEB, USA). Index-coded sample clustering was performed on a cBot Cluster Generation System using a TruSeq PE Cluster Kit v3-cBot-HS (Illumina), according to the manufacturer’s instructions. After cluster generation, the libraries were sequenced on an Illumina Novaseq platform, and 150 bp paired-end reads were generated.
Transcriptomic data analyses
Adapter sequences and low-quality reads were removed from the raw sequencing reads using Fastp (v0.23.1)22. The resulting cleaned reads were aligned to the GRCh38 human reference genome using HISAT2 (v2.0.5)23. Aligned reads were assembled in a reference-guided manner using StringTie (v1.3.3b)24. Gene-level read counts were quantified with featureCounts (v1.5.0-p3) using the GENCODE gene annotation (v21)25.
Differentially expressed genes (DEGs) between KC and normal samples were identified using the DESeq2 R package (v1.20.0)26. P-values were adjusted using the Benjamini–Hochberg method to control for false discoveries. Genes with adjusted P-values < 0.05 and absolute fold changes (FCs) ≥ 2 were considered differentially expressed genes. Functional enrichment of Gene Ontology (GO) terms and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways among the DEGs were determined using the clusterProfiler R package (v4.6.0)27. GO terms and KEGG pathways with an adjusted P-value < 0.05 were considered significantly enriched. Immune cell infiltration differences between KC and controls were assessed using the xCell algorithm (v1.1.0)28. In addition to DEG-based enrichment analysis, gene set enrichment analysis (GSEA) was performed using clusterProfiler (v4.6.0) to compare KC subgroups. Genes were ranked based on the absolute value of the log2FC, and enrichment analyses were performed using the gseGO and gseKEGG functions. An adjusted P-value < 0.05 was set as the threshold for statistical significance. All software and functions described above were run using default parameters.
Weighted gene co-expression network analyses (WGCNAs) (v1.72-1) was applied to compare KC and normal samples, as well as to compare different pairs of KC subgroups29. A scale-free network was constructed using the blockwiseModules function and an adaptive soft-thresholding approach based on the scale-free topology fit index calculated over a power range of 1–20. The optimal power was determined by linear regression (powerEstimate); if automatic selection failed, a default power of 6 was applied. Pearson correlation coefficients were calculated between module eigengenes and trait values, and modules with an absolute correlation > 0.5 and P < 0.05 were considered significantly associated with the trait of interest. DEGs were intersected with genes from trait-associated modules to identify relevant candidate genes, and GO and KEGG analyses of the overlapping genes were performed. Hub genes were first selected as the top 20 genes with the highest connectivity in each module and were then ranked according to their maximal clique centrality values.
Protein-protein interaction networks were constructed using the STRING database with a minimum interaction score threshold of 0.430. The resulting networks were visualized using Cytoscape v3.10.031. To identify key genes associated with KC, hub genes from WGCNA modules were intersected with DEGs between KC and control groups. For KC subgroups, genes common to both WGCNA modules and DEGs were further analyzed using STRING and CytoHubba32 to identify and visualize the top 10 nodes.
Quantitative real-time polymerase chain reaction (qRT-PCR)
The qRT-PCR validation was performed using eight KC corneas and four control corneas; two control corneas were part of the RNA-seq cohort. Complementary DNA (cDNA) was synthesized using the iScript™ gDNA Clear cDNA Synthesis Kit (Bio-Rad, USA). Fifteen DEGs with low p-values, high FCs, and relatively stable expression levels across samples within each group were selected, including five key genes associated with KC and five associated with KC subgroups. The primer sequences for these genes are listed in Table S1. qRT-PCR was carried out using five-fold diluted cDNA and the SYBR Green Pro Taq HS qRT-PCR Kit (AG11701, Accurate Biotechnology, China) in a 20 µL reaction volume containing 10 µL 2X SYBR Green Pro Taq HS Premix, 0.4 µL each of forward and reverse primers (10 µM), and RNase-free water. Amplification was performed on the Gentier 96R Real-Time PCR System (Tianlong, China) using the following thermal cycling conditions: initial denaturation at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. Melting curve analysis was conducted to confirm specificity. Relative gene expression was quantified using the 2−ΔΔCt method, with GAPDH as the housekeeping gene. Statistical analysis was conducted using unpaired two-tailed Student’s t-tests in R (version 4.4.3, R Foundation for Statistical Computing, Vienna, Austria). P < 0.05 was considered significant.
Validation of key genes in public transcriptomic datasets
Three transcriptome profiling datasets related to KC, including GSE20479133, GSE15163134, and GSE7793835, were obtained from the Gene Expression Omnibus database. Detailed sample information is provided in Table S2. Expression values for 15 previously selected key genes used in qRT-PCR validation were extracted from each dataset and normalized to fragments per kilobase of transcript per million mapped reads. A small expression matrix consisting of these 15 genes was constructed for each dataset. Differential expression analysis between KC and control groups was performed using the DESeq2 package in R. Genes with adjusted P-values < 0.05 were considered differentially expressed. Receiver operating characteristic curve analysis was subsequently conducted to evaluate the discriminatory power of each gene in distinguishing KC samples from controls.
Results
Clinical characteristics of patients
The study included 31 corneas from patients with KC (K1–K31) and 4 corneas from control donors (N1–N4). The demographic and clinical characteristics of participants with KC and normal control donors are summarized in Table S3. None of the participants reported a family history of KC.
Sequencing quality and annotation results
Transcriptome sequencing of the 35 corneal stroma samples generated 1,649,180,812 raw reads (Table 1). After quality control (QC), each sample retained an average of 46,244,052 QC-passed reads, and the Q20 and Q30 values were 97.18% and 92.93%, respectively. The average GC content was 49.19%. An average of 94.67% of QC-passed reads were mapped to the reference human genome; 90.48% were mapped to a single location, and 4.19% were mapped to multiple locations. The KC samples and normal tissues were separated on the principal component analysis (Fig. 1A). Figure 1B shows the correlation of gene expression profiles across all samples.
Table 1.
Summary of RNA-seq data.
| Sample | Raw reads | Raw data (G) | QC-passed reads | QC-passed data (G) | Q30 (%) | Q20 (%) | GC (%) | Total mapped (%) | Unique mapped (%) |
|---|---|---|---|---|---|---|---|---|---|
| K1 | 46,914,974 | 7.04 | 46,019,596 | 6.90 | 98.83 | 96.65 | 47.84 | 94.85 | 88.86 |
| K2 | 46,112,884 | 6.92 | 45,360,216 | 6.80 | 98.88 | 96.83 | 49.31 | 90.20 | 84.15 |
| K3 | 42,669,924 | 6.40 | 41,624,946 | 6.24 | 98.74 | 96.48 | 48.01 | 95.15 | 89.08 |
| K4 | 49,863,610 | 7.48 | 48,949,144 | 7.34 | 98.95 | 96.93 | 48.85 | 93.88 | 87.85 |
| K5 | 49,732,230 | 7.46 | 48,566,014 | 7.28 | 98.89 | 96.77 | 48.89 | 93.51 | 88.29 |
| K6 | 52,610,592 | 7.89 | 51,797,224 | 7.77 | 98.95 | 96.97 | 48.69 | 93.89 | 89.76 |
| K7 | 45,529,138 | 6.83 | 44,795,676 | 6.72 | 98.94 | 96.89 | 49.70 | 94.42 | 90.63 |
| K8 | 45,388,284 | 6.81 | 44,699,232 | 6.70 | 98.87 | 96.79 | 49.52 | 95.28 | 89.75 |
| K9 | 45,290,792 | 6.79 | 44,624,598 | 6.69 | 98.99 | 97.03 | 49.55 | 92.72 | 88.83 |
| K10 | 46,529,664 | 6.98 | 45,139,296 | 6.77 | 98.92 | 95.69 | 49.53 | 93.70 | 89.78 |
| K11 | 43,653,064 | 6.55 | 42,876,794 | 6.43 | 98.96 | 96.92 | 49.55 | 92.57 | 88.46 |
| K12 | 46,884,442 | 7.03 | 46,192,470 | 6.93 | 98.86 | 96.81 | 49.11 | 93.89 | 86.53 |
| K13 | 46,476,636 | 6.97 | 46,068,992 | 6.91 | 97.53 | 93.44 | 48.98 | 95.96 | 92.74 |
| K14 | 42,146,804 | 6.32 | 41,713,934 | 6.26 | 97.59 | 93.52 | 48.74 | 95.18 | 92.50 |
| K15 | 50,216,930 | 7.53 | 49,734,466 | 7.46 | 97.51 | 93.38 | 48.37 | 96.60 | 93.64 |
| K16 | 47,518,718 | 7.13 | 47,138,692 | 7.07 | 97.53 | 93.44 | 48.46 | 96.58 | 93.62 |
| K17 | 47,108,778 | 7.07 | 45,342,348 | 6.80 | 99.05 | 96.15 | 50.29 | 95.23 | 91.48 |
| K18 | 46,208,964 | 6.93 | 45,676,422 | 6.85 | 97.18 | 92.93 | 49.78 | 95.69 | 85.74 |
| K19 | 45,930,334 | 6.89 | 45,537,536 | 6.83 | 97.52 | 93.46 | 49.32 | 96.25 | 92.24 |
| K20 | 48,221,206 | 7.23 | 47,744,094 | 7.16 | 97.63 | 93.68 | 49.08 | 95.09 | 91.96 |
| K21 | 46,129,352 | 6.92 | 45,746,626 | 6.86 | 97.44 | 93.25 | 49.74 | 95.51 | 92.54 |
| K22 | 45,650,462 | 6.85 | 45,151,908 | 6.77 | 97.35 | 93.16 | 48.84 | 95.90 | 93.23 |
| K23 | 47,112,486 | 7.07 | 45,561,250 | 6.83 | 99.10 | 96.16 | 50.01 | 96.15 | 92.38 |
| K24 | 47,713,950 | 7.16 | 46,129,880 | 6.92 | 99.11 | 96.24 | 50.20 | 95.07 | 90.83 |
| K25 | 55,790,908 | 8.37 | 54,006,952 | 8.10 | 99.04 | 95.99 | 50.04 | 94.09 | 90.21 |
| K26 | 47,709,388 | 7.16 | 46,006,100 | 6.90 | 99.12 | 96.25 | 50.12 | 93.95 | 90.81 |
| K27 | 50,598,326 | 7.59 | 48,862,274 | 7.33 | 99.01 | 96.06 | 49.36 | 95.78 | 92.48 |
| K28 | 46,615,036 | 6.99 | 45,912,674 | 6.89 | 97.54 | 93.49 | 49.44 | 95.99 | 93.24 |
| K29 | 40,985,178 | 6.15 | 40,103,834 | 6.02 | 98.70 | 96.48 | 49.39 | 89.50 | 84.54 |
| K30 | 47,655,402 | 7.15 | 47,058,096 | 7.06 | 98.09 | 94.69 | 49.38 | 97.04 | 94.23 |
| K31 | 46,162,270 | 6.92 | 45,442,676 | 6.82 | 98.12 | 94.64 | 48.36 | 96.98 | 94.25 |
| N1 | 53,036,620 | 7.96 | 52,324,626 | 7.85 | 98.98 | 97.00 | 48.62 | 94.48 | 90.65 |
| N2 | 47,221,754 | 7.08 | 46,493,130 | 6.97 | 98.96 | 96.93 | 48.44 | 94.24 | 90.61 |
| N3 | 45,783,732 | 6.87 | 45,023,052 | 6.75 | 98.94 | 96.89 | 48.84 | 94.92 | 91.57 |
| N4 | 46,007,980 | 6.90 | 45,138,052 | 6.77 | 98.95 | 96.96 | 49.44 | 93.14 | 89.24 |
QC quality control.
Fig. 1.
Overview of transcriptomic analysis of KC and normal samples. (A) Principal component analysis illustrating the separation between KC and normal samples. (B) Pearson correlation heatmap displaying inter-sample correlations. (C) Volcano plot showing DEGs between samples from patients with KC and normal control patients. (D) KEGG pathway enrichment analysis of DEGs. KEGG imagery adapted from Kanehisa Laboratories36. (E) GO enrichment analysis showing the top 20 significantly enriched terms, including biological processes (BP), cellular components (CC), and molecular functions (MF). KC = keratoconus; pc1/pc2 = principal component 1/2; Padj = adjusted P-value; FDR = false discovery rate.
Differential gene expression and functional enrichment analysis
Comparative analysis of KC and normal samples revealed 4469 DEGs, comprising 1254 upregulated and 3215 downregulated genes (Padj < 0.05) (Fig. 1C; Table S4). GO analysis showed that biological processes related to cell adhesion, signal transduction, ECM organization, axon guidance, nervous system development, neuron migration, cell migration, inflammatory response, and cell population proliferation were significantly downregulated in the KC samples. ECM constituents, cell adhesion elements, cell junctions, and synaptic structures were significantly downregulated cellular components in the KC samples. The molecular functions, including ECM structural constituents, binding interactions, signal transduction, ion channel activity, cytokine receptor activity, and cytokine binding, were downregulated in the KC samples (Padj < 0.05) (Fig. 1E). Immune microenvironment analysis revealed reduced dendritic cell (DC) infiltration in KC stroma (false discovery rate < 0.05) (Fig S1). The top 20 enriched KEGG pathways in the KC samples compared with the normal samples included cell adhesion, focal adhesion, ECM–receptor interaction, axon guidance, regulation of transient receptor potential (TRP) channels by inflammatory mediators, Wnt, Hippo, calcium, cGMP-PKG, apoptosis, cell cycle arrest, and efferocytosis pathways (Padj < 0.05) (Fig. 1D).
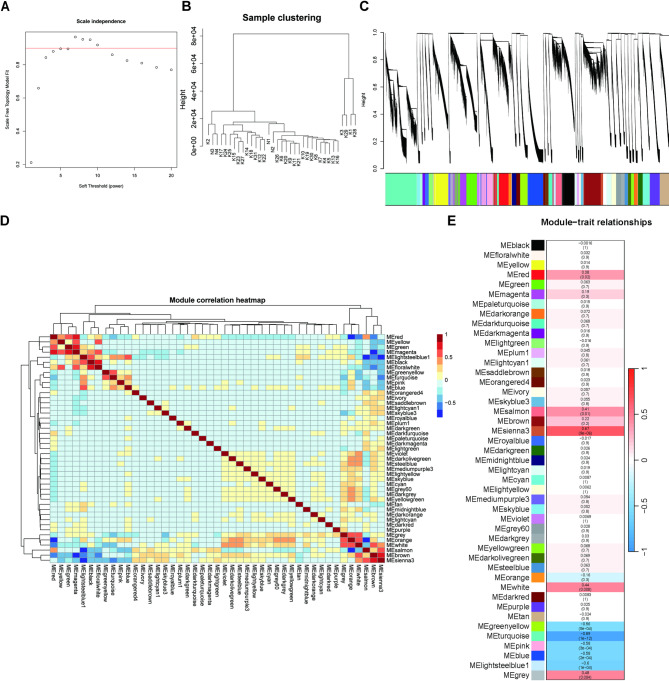
WGCNA of KC versus normal cornea
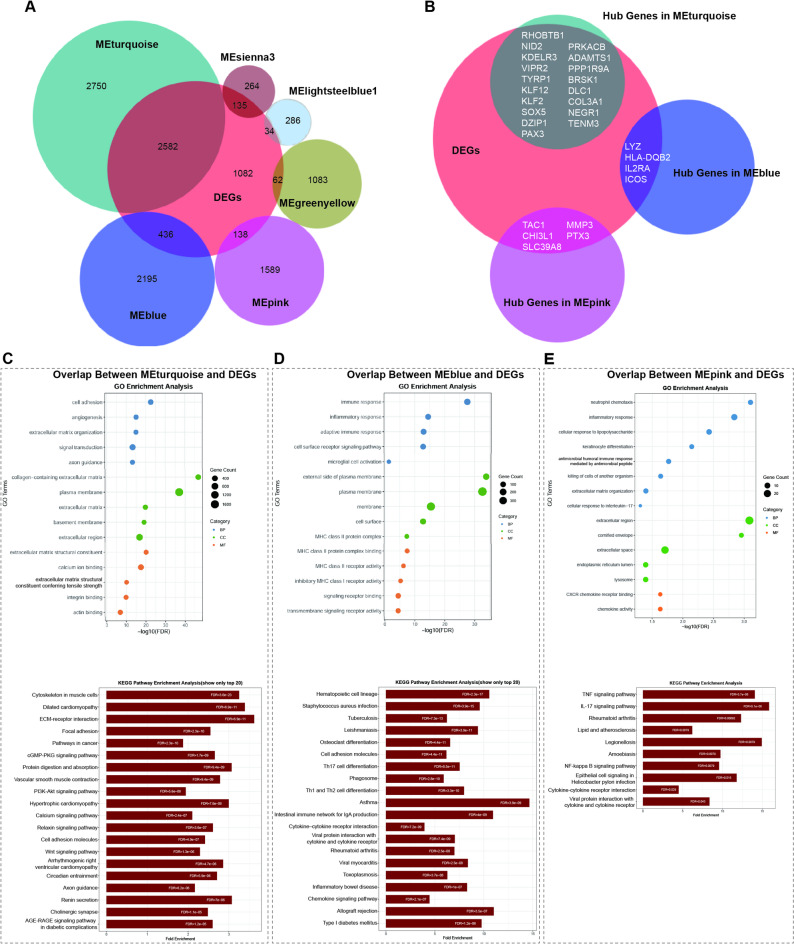
The soft-thresholding power for achieving scale-free topology and the resulting cluster dendrogram are shown in Fig. 2A, B, respectively. Forty-five gene modules were identified (Fig. 2C, D), and the hub genes for each module are listed in Table S5. Among these, the MEturquoise, MEsienna3, MElightsteelblue1, MEblue, MEpink, and MEgreenyellow modules showed significant correlations with KC (P < 0.05) (Fig. 2E). The overlap between DEGs and module genes is shown in Fig. 3A. Functional enrichment analysis of the overlapping genes revealed that the MEturquoise, MEblue, and MEpink modules were significantly enriched. Integration of hub genes from these three modules with DEGs resulted in 28 signature genes (Fig. 3B). The MEturquoise module was enriched for terms and KEGG pathways related to ECM organization, signaling pathways, neurodevelopment, and synaptic regulation (Padj < 0.05) (Fig. 3C), and the MEblue and MEpink modules were enriched for immune and inflammatory processes (Padj < 0.05) (Fig. 3D, E).
Fig. 2.
WGCNA of KC and normal samples. (A) Diagnostic plot for selecting the soft-thresholding power to construct a scale-free network. (B) Hierarchical clustering dendrogram of samples. (C) Gene dendrogram clustered by topological overlap measures, with color-coded modules identified via dynamic tree cutting. (D) Pairwise correlation heatmap of module eigengenes. (E) Module–trait association heatmap quantifying relationships between module eigengenes and clinical traits (KC vs. normal). Each cell shows the Pearson correlation coefficient (top) and the corresponding P-value (bottom, in parentheses).
Fig. 3.
Enrichment analysis of key modules and identification of candidate hub genes in KC vs. normal samples. (A) Venn diagram showing the overlap between DEGs and genes from the six significantly correlated WGCNA modules. (B) Venn diagram displaying the intersection between DEGs and hub genes in the MEturquoise, MEblue, and MEpink modules. (C–E) Functional enrichment analysis of overlapping genes in the (C) MEturquoise, (D) MEblue, and (E) MEpink modules, with GO terms shown in the upper panels and KEGG pathways in the lower panels. KEGG imagery adapted from Kanehisa Laboratories36. BP = biological processes; CC = cellular components; MF = molecular functions; FDR = false discovery rate.
GSEA of clinical risk factor-based subgroups
GSEA of five subgroups was conducted to identify significantly enriched GO terms and KEGG pathways associated with KC risk factors (Padj < 0.05). ECM organization and ECM constituent components were among the most enriched GO terms in the elevated ERI subgroup, and KEGG analysis revealed enrichment in the focal adhesion, ECM–receptor interaction, and PI3K-Akt signaling pathways (Fig. 4A). In the high BMI subgroup, the enriched GO terms indicated the downregulation of mitochondrial-related pathways, including the electron transport chain and adenosine triphosphate (ATP) synthesis, and ribosomal and translation-associated processes. KEGG analysis confirmed the downregulation of the oxidative phosphorylation and ribosomal pathways in the high BMI subgroup (Fig. 4B). In patients with KC and low childhood SES, the enriched GO terms indicated upregulation of immune activation, mitochondrial metabolism, and ATP synthesis pathways. The KEGG pathways enriched in the low childhood SES subgroup included oxidative phosphorylation and ribosome-related processes (Fig. 4C).
Fig. 4.
GSEA of KC subgroups. (A–C) GSEA results for KC subgroups stratified by (A) ERI, (B) BMI, and (C) childhood SES, with GO terms shown in the upper panels and KEGG pathways in the lower panels. KEGG imagery adapted from Kanehisa Laboratories36. Padj = adjusted P-value; BP = biological processes; CC = cellular components; MF = molecular functions.
In the sex-specific analysis, the chromatin-modifying protein adaptor activity, sex chromosome dosage compensation, and antioxidant activity GO terms were enriched in females, and the histone demethylase activity and androgen receptor binding GO terms were enriched in males (Fig S2A). No KEGG pathways were significantly enriched according to sex. In the allergy subgroup, enriched GO terms included molecular carrier activity, regulation of respiratory burst, histone chaperone activity, and response to hydrogen peroxide (Fig S2B). The spliceosome KEGG pathway was also significantly enriched in the allergy subgroup.
WGCNA of KC subgroups
WGCNA identified 40 gene modules within the KC group, and the hub genes for these modules are listed in Table S6. The MEyellowgreen module significantly correlated with ERI (Fig. 5A), and the MElightyellow module significantly correlated with BMI (Fig. 5B). The MElightyellow, MEyellow, and MEgrey modules were associated with childhood SES (P < 0.05) (Fig. 5C). No significant associations with the sex or allergy subgroups were detected (Fig S2C and D). The overlap between DEGs identified between KC and normal samples and the subgroup-related modules is shown in Fig. 5D.
Fig. 5.
WGCNA of KC subgroups. (A–C) Heatmaps of module–trait associations showing the correlation between module eigengenes and clinical traits, including (A) ERI, (B) BMI, and (C) childhood SES. Each cell shows the Pearson correlation coefficient (top) and the corresponding P-value (bottom, in parentheses). (D) Venn diagram illustrating the overlap between DEGs from the KC vs. normal comparison and genes from four significantly correlated modules. (E) GO enrichment analysis of overlapping genes in the MEyellowgreen module. (F, G) KEGG pathway enrichment analysis of overlapping genes in the (F) MEyellowgreen and (G) MEgrey modules. KEGG imagery adapted from Kanehisa Laboratories36. (H, I) Top 10 hub genes identified from the overlapping genes in the (H) MEyellowgreen and (I) MEgrey modules. CC = cellular components; MF = molecular functions; FDR = false discovery rate.
Functional enrichment analysis revealed that the MEyellowgreen module was enriched in ECM-related processes and Wnt and PI3K-Akt signaling pathways (Padj < 0.05) (Fig. 5E, F). The MEgrey module was enriched in the cholinergic synapse, axon guidance, and inflammatory mediator regulation of TRP channels (Padj < 0.05) (Fig. 5G). The top 10 hub genes among the overlapping genes in the MEyellowgreen and MEgrey modules, identified by CytoHubba based on STRING analysis, are shown in Fig. 5H and I, respectively.
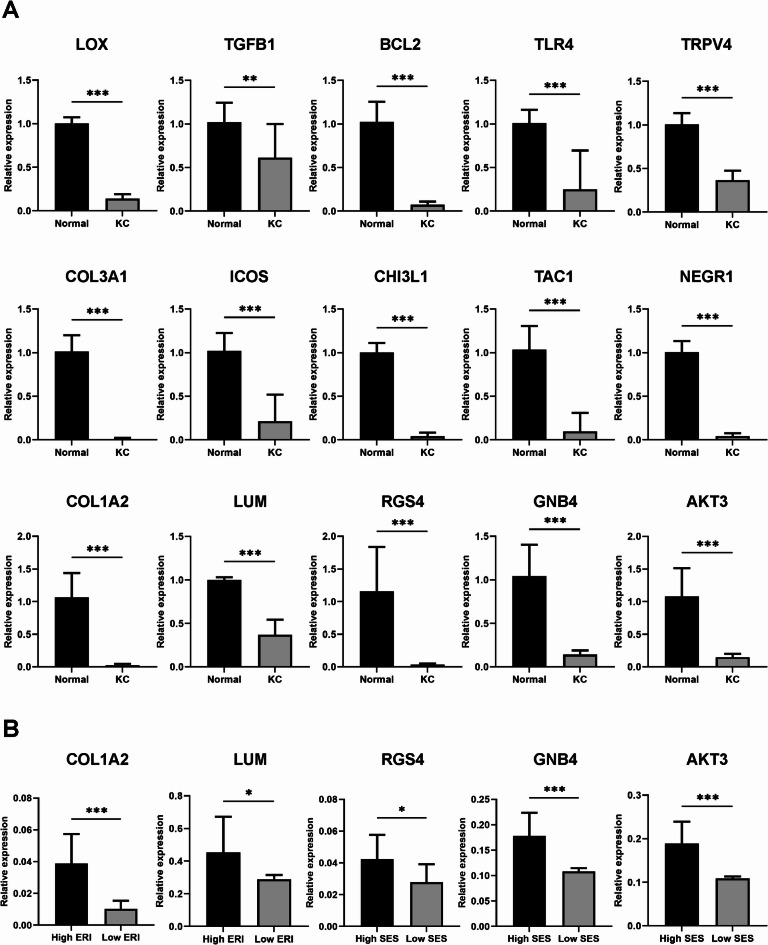
Validation of gene expression patterns using qRT-PCR
RNA-Seq expression patterns of 15 selected genes were validated by qRT-PCR. All of these genes, including LOX, TGFB1, BCL2, TLR4, TRPV4, and five key genes associated with KC (COL3A1, ICOS, CHI3L1, TAC1, and NEGR1), as well as five genes linked to KC subgroups (COL1A2, LUM, RGS4, GNB4, and AKT3) were significantly downregulated in KC patients compared with normal controls (P < 0.05) (Fig. 6).
Fig. 6.
Analysis of 15 genes using qRT-PCR to validate the RNA-Seq data. (A) Comparison of gene expression between KC patients and normal controls. (B) Comparison among KC subgroups. * P < 0.05, ** P < 0.01, *** P < 0.001. qRT-PCR = quantitative real-time polymerase chain reaction; KC = keratoconus; ERI = eye rubbing intensity; SES = socioeconomic status.
We further analyzed the expression of the two ERI-associated genes and three SES-associated genes across patient subgroups. Among the eight KC patients, the four with high ERI exhibited significantly lower expression levels of COL1A2 and LUM compared with the four with low ERI. Additionally, RGS4, GNB4, and AKT3, which are associated with SES, were significantly downregulated in the four patients with low SES (P < 0.05).
Validation of gene expression patterns using public transcriptomic datasets
The expression of the 15 genes was further validated using three public transcriptomic datasets. All 15 genes were downregulated in at least one dataset, with statistical significance (Padj < 0.05) (Fig. 7). The receiver operating characteristic curves showed that each gene achieved an area under the curve of 0.827 or higher in at least one dataset, indicating strong discriminative potential (Fig S3).
Fig. 7.
Violin plots showing expression patterns of 15 genes in three public transcriptomic datasets. (A) GSE204791, (B) GSE151631, and (C) GSE77938. # Padj < 0.05. KC = keratoconus.
Discussion
KC is a multifactorial disease with complex etiology, stage-specific phenotypes, and multiple pathological pathways. Its molecular features across different etiologies and stages remain unclear. Although traditionally considered non-inflammatory, KC is increasingly associated with immune dysregulation. Prior studies reported elevated inflammatory mediators37; however, our transcriptomic analysis of late-stage KC (mean keratometry > 55.0 D; awaiting transplantation) revealed marked suppression of innate and adaptive immunity, reduced migratory signaling, and impaired ECM remodeling. These findings are consistent with spatial transcriptomic studies indicating that central corneal inflammation suppresses inflammatory activity in the peripheral stromal38. This pattern is supported by the “doughnut-shaped” epithelial response in which wound-response genes are upregulated centrally but downregulated in the periphery39. Thus, inflammatory dynamics are spatially distinct, and the anti-inflammatory signature detected in our bulk transcriptomic data likely reflects peripheral stromal quiescence. In advanced KC, the peripheral cornea may have progressed past inflammation and entered a post-inflammatory, quiescent state38. In this context, reduced ECM remodeling and cell motility and the antiproliferative and pro-apoptotic phenotypes likely reflect a shift toward tissue stabilization rather than active repair40.
Our results also reveal the significant downregulation of genes involved in synaptic function, neurotransmitters (including neuropeptides, neurotrophins, glutamate, acetylcholine, and dopamine), and axon guidance. The downregulation of TAC1 and NEGR1 was validated using qRT-PCR and public datasets. These results suggest that neurogenic and neuroinflammatory mechanisms contribute to KC, consistent with clinical observations of reduced nerve density and neurodegeneration in patients41. Corneal wound healing relies on reciprocal interactions between keratocytes and corneal nerves: keratocytes secrete neurotrophins that promote nerve survival and differentiation, while nerves release mediators such as nerve growth factor to stimulate inflammation42. However, chronic neuroinflammation disrupts this feedback loop, leading to neurotrophic loss and impaired neural and stromal regeneration43. Collectively, these findings support a biphasic model of KC, in which early disease is characterized by inflammation-driven repair, whereas advanced disease is marked by reduced nerve density, diminished neurotrophic signaling, and progressive neuronal–stromal degeneration.
While prior studies emphasized stromal and immune interactions44, our data highlight neuron–immune–stromal dysregulation, especially in late-stage KC. KEGG analysis identified the “inflammatory mediator regulation of TRP channels” pathway, a little-studied mechanism in KC. TRP channels, expressed in corneal nerves, epithelial, stromal, endothelial cells, and DCs, mediate mechanical, thermal, and nociceptive responses and facilitate crosstalk among these compartments through neuropeptides, cytokines, and electrical signals45. Their activation promotes repair but can also drive inflammation and fibrosis46. For example, TRPV4 integrates mechanical stimuli and soluble signals to amplify inflammatory signaling in stiffer environments47,48. In KC, TRP downregulation was associated with reduced DC infiltration, chemokine, and Toll-like receptor expression, suggesting impaired neuroimmune signaling. Reduced TRPV4 and TLR4 expression was further validated by qRT-PCR and public datasets. These results support a model where early inflammation alters ECM biomechanics and neuroimmune crosstalk, progressing to immune tolerance and dysregulated stromal repair, partly mediated by TRP dysfunction. Signature genes further implicate immune dysregulation (LYZ, IL2RA, ICOS, CHI3L1), ECM remodeling (MMP3, COL3A1, NID2, ADAMTS1), and neuro-signaling damage (TAC1, VIPR2, PRKACB, BRSK1), confirming neuron–immune–stromal dysregulation in KC.
Subgroup analyses revealed that strong eye rubbing inhibited structural maintenance, tissue reconstruction, and the PI3K-Akt signaling pathway, which regulates corneal cell proliferation, apoptosis, and migration49. Enrichment of the yellowgreen module highlighted ECM–receptor interaction, cell adhesion, focal adhesion, and Wnt signaling, which is key to mechanotransduction50. Ten key module genes, primarily associated with ECM organization and tissue integrity, included COL1A2 and LUM, whose downregulation in KC, as well as further reduction in high-ERI KC patients, was confirmed by qRT-PCR. These results suggest that excessive eye rubbing worsens KC by disrupting ECM structure, remodeling, antiproliferative and hyperapoptotic signaling, and mechanotransduction.
GSEA revealed downregulation of mitochondrial pathways, including mitochondrial assembly, ATP synthesis, the electron transport chain, and oxidative phosphorylation, in KC patients with high BMIs, indicating mitochondrial dysfunction and potential energy deficits. Suppression of ribosomal and translation pathways suggests reduced protein synthesis, likely due to energy constraints. These findings imply that elevated BMIs may exacerbate mitochondrial impairment, disrupt metabolic homeostasis, increase cellular stress, and promote mitochondrial-mediated apoptosis in KC51.
WGCNA identified a module exhibiting downregulation of cholinergic synapse and axon guidance pathways in KC patients with low childhood SES. This module includes RGS2/4, GNB4, GNAZ, GNG7, ADCY9, PIK3R3, AKT3, and PLCB1, key regulators of neuronal signaling, synaptic plasticity, and cell migration. Downregulation of RGS4, GNB4, and AKT3 was validated, with higher expression observed in high-SES KC patients compared with low-SES patients. These findings align with studies linking low SES to impaired neurodevelopment, likely mediated by chronic psychosocial stress52. In contrast, environmental enrichment associated with high SES upregulates genes involved in synapse formation and maintenance53. GSEA-GO analysis of low-SES patients revealed upregulation of immune activation and mitochondrial metabolism, consistent with the established association between low SES, chronic inflammation, and increased disease susceptibility54. Overall, low SES may contribute to neuron–immune–stromal dysregulation in KC.
Sex-specific differences in chromatin regulation were detected. Chromatin–protein adaptor activity was upregulated in females, and histone demethylase activity was increased in males, highlighting the distinct epigenetic regulatory landscapes55. These differences may underlie sex-specific susceptibility or progression of KC. Females also showed higher antioxidant activity, potentially enhancing corneal defense,56 whereas males exhibited increased androgen receptor signaling and nuclear androgen receptor binding, whose roles in KC remain unclear. Subgroup analysis by allergy status revealed few enriched pathways, no consistent expression patterns, and no significant WGCNA modules, indicating that allergy status has minimal impact on transcriptomic variation in this cohort.
This study has several limitations. The absence of spatial transcriptomics and single-cell RNA sequencing limited resolution, preventing detailed characterization of regional and cell-type-specific corneal changes. Focusing on a single disease stage may underrepresent transcriptomic variability across progression. Given the observational design, associations identified between sociodemographic and behavioral factors and gene expression are correlational and do not establish causality. Findings from this specific cohort may not generalize to broader populations, and sociodemographic measures like SES may lack sufficient granularity to capture complex influences on disease. Future studies should use higher-resolution transcriptomics, longitudinal or experimental designs, and more detailed sociodemographic data to clarify causal mechanisms and disease dynamics.
Despite these limitations, we identified significant transcriptomic differences between late-stage KC and normal cornea, and linked demographic and lifestyle factors to KC-related gene expression. These findings provide a molecular framework for understanding how environmental and social disparities may influence KC pathogenesis, warranting further investigation.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients, donors, and their families who, by providing research consent, made this study possible.
Author contributions
D.K., X.G., and Q.Y. wrote the main manuscript text. D.K., P.R., and H.L. collected and managed the data. P.R. and H.J. designed the study. D.K., X.G., and Q.Y. conducted the study. All authors reviewed and approved the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81970768).
Data availability
The datasets generated and analyzed during the current study are available in the National Center for Biotechnology Information Sequence Read Archive repository under BioProject accession number PRJNA1303973.
Code availability
The code that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Written informed consent was obtained from all patients or the legal guardians of individuals under 18 years and the control donors’ next-of-kin for research purposes. The study protocol was approved by the Medical Science Research Ethics Committee of Peking University Third Hospital (Protocol No. M2023859) and adhered strictly to the principles of the Declaration of Helsinki.
Consent for publication
Consent for publication was not required as the data are fully anonymized and no identifiable individual information is included.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mas Tur, V., MacGregor, C., Jayaswal, R., O’Brart, D. & Maycock, N. A review of keratoconus: Diagnosis, pathophysiology, and genetics. Surv. Ophthalmol.62, 770–783. 10.1016/j.survophthal.2017.06.009 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Gokhale, N. S. Epidemiology of keratoconus. Indian J. Ophthalmol.61, 382–383. 10.4103/0301-4738.116054 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lucas, S. E. M. & Burdon, K. P. Genetic and environmental risk factors for keratoconus. Annu. Rev. Vis. Sci.6, 25–46. 10.1146/annurev-vision-121219-081723 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Monteiro de Barros, M. R. & Chakravarti, S. Pathogenesis of keratoconus: NRF2-antioxidant, extracellular matrix and cellular dysfunctions. Exp. Eye Res.219, 109062. 10.1016/j.exer.2022.109062 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hashemi, H. et al. The prevalence and risk factors for keratoconus: A systematic review and meta-analysis. Cornea39, 263–270. 10.1097/ICO.0000000000002150 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Yang, K. et al. Independent and interactive effects of eye rubbing and atopy on keratoconus. Front. Immunol.13, 999435. 10.3389/fimmu.2022.999435 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eliasi, E. et al. The association between keratoconus and body mass index: A population-based cross-sectional study among half a million adolescents. Am. J. Ophthalmol.224, 200–206. 10.1016/j.ajo.2020.11.021 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Ahmad, T. R. et al. Socioeconomic correlates of keratoconus severity and progression. Cornea42, 60–65. 10.1097/ICO.0000000000002993 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loukovitis, E. et al. The proteins of keratoconus: A literature review exploring their contribution to the pathophysiology of the disease. Adv. Ther.36, 2205–2222. 10.1007/s12325-019-01026-0 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cai, J., Estes, A. & Liu, Y. Omics analyses in keratoconus: From transcriptomics to proteomics. Curr. Ophthalmol. Rep.8, 216–225. 10.1007/s40135-020-00253-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy, S. et al. Interplay between hereditary and environmental factors to establish an in vitro disease model of keratoconus. Drug Discov Today24, 403–416. 10.1016/j.drudis.2018.10.017 (2019). [DOI] [PubMed] [Google Scholar]
- 12.Khaled, M. et al. (ed, L.) Molecular and histopathological changes associated with keratoconus. Biomed. Res. Int.2017 7803029 10.1155/2017/7803029 (2017). [DOI] [PMC free article] [PubMed]
- 13.Shetty, R. et al. Biochemical markers and alterations in keratoconus. Asia Pac J. Ophthalmol.9, 533–540. 10.1097/apo.0000000000000332 (2020). [DOI] [PubMed] [Google Scholar]
- 14.Chang, S. H. et al. Machine Learning-Driven transcriptome analysis of keratoconus for predictive biomarker identification. Biomedicines1310.3390/biomedicines13051032 (2025). [DOI] [PMC free article] [PubMed]
- 15.Lyu, N. et al. Multi-dataset identification of innovative feature genes and molecular mechanisms in keratoconus. J. Cell. Mol. Med.28, e70079. 10.1111/jcmm.70079 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niu, X., Xu, M., Zhu, J., Zhang, S. & Yang, Y. Identification of the immune-associated characteristics and predictive biomarkers of keratoconus based on single-cell RNA-sequencing and bulk RNA-sequencing. Front. Immunol.14, 1220646. 10.3389/fimmu.2023.1220646 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macé, M. et al. Comparative transcriptome and network biology analyses demonstrate antiproliferative and hyperapoptotic phenotypes in human keratoconus Corneas. Invest. Ophthalmol. Vis. Sci.52, 6181–6191. 10.1167/iovs.10-70981 (2011). [DOI] [PubMed] [Google Scholar]
- 18.Jaskiewicz, K. et al. Non-allergic eye rubbing is a major behavioral risk factor for keratoconus. PLoS One18, e0284454. 10.1371/journal.pone.0284454 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romano, V. et al. Quality assurance in corneal transplants: Donor cornea assessment and oversight. Surv. Ophthalmol.69, 465–482. 10.1016/j.survophthal.2023.12.002 (2024). https://doi.org:. [DOI] [PubMed] [Google Scholar]
- 20.Pan, X. F., Wang, L. & Pan, A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol.9, 373–392. 10.1016/s2213-8587(21)00045-0 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Boylan, J. M., Cundiff, J. M., Jakubowski, K. P., Pardini, D. A. & Matthews, K. A. Pathways linking childhood SES and adult health behaviors and psychological resources in black and white men. Ann. Behav. Med.52, 1023–1035. 10.1093/abm/kay006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen, S., Zhou, Y., Chen, Y. & Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics34, i884–i890. 10.1093/bioinformatics/bty560 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol.37, 907–915. 10.1038/s41587-019-0201-4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pertea, M. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol.33, 290–295. 10.1038/nbt.3122 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liao, Y., Smyth, G. K. & Shi, W. FeatureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics30, 923–930. 10.1093/bioinformatics/btt656 (2014). [DOI] [PubMed] [Google Scholar]
- 26.Love, M. I., Huber, W. & Anders, S. Moderated Estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol.15, 550. 10.1186/s13059-014-0550-8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu, G., Wang, L. G., Han, Y. & He, Q. Y. ClusterProfiler: An R package for comparing biological themes among gene clusters. OMICS16, 284–287. 10.1089/omi.2011.0118 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aran, D., Hu, Z. & Butte, A. J. xCell: Digitally portraying the tissue cellular heterogeneity landscape. Genome Biol.18, 220. 10.1186/s13059-017-1349-1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langfelder, P. & Horvath, S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinform.9, 559. 10.1186/1471-2105-9-559 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Szklarczyk, D. et al. The STRING database in 2025: Protein networks with directionality of regulation. Nucleic Acids Res.53, D730–D737. 10.1093/nar/gkae1113 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shannon, P. et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res.13, 2498–2504 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chin, C. H. et al. CytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol.8(Suppl 4). 10.1186/1752-0509-8-s4-s11 (2014). [DOI] [PMC free article] [PubMed]
- 33.Stachon, T. et al. Altered regulation of mRNA and MiRNA expression in epithelial and stromal tissue of keratoconus Corneas. Invest. Ophthalmol. Vis. Sci.63, 7. 10.1167/iovs.63.8.7 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shinde, V. et al. RNA sequencing of Corneas from two keratoconus patient groups identifies potential biomarkers and decreased NRF2-antioxidant responses. Sci. Rep.10, 9907. 10.1038/s41598-020-66735-x (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kabza, M. et al. Collagen synthesis disruption and downregulation of core elements of TGF-β, Hippo, and Wnt pathways in keratoconus Corneas. Eur. J. Hum. Genet.25, 582–590. 10.1038/ejhg.2017.4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanehisa, M., Furumichi, M., Sato, Y., Matsuura, Y. & Ishiguro-Watanabe, M. KEGG: Biological systems database as a model of the real world. Nucleic Acids Res.53, D672–d677. 10.1093/nar/gkae909 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Santodomingo-Rubido, J. et al. Keratoconus: An updated review. Cont. Lens Anterior Eye45, 101559. 10.1016/j.clae.2021.101559 (2022). [DOI] [PubMed] [Google Scholar]
- 38.Luo, S. et al. Spatial transcriptomics and single-cell RNA-sequencing revealed dendritic cell-mediated inflammation in keratoconus. Ocul Surf.36, 134–150. 10.1016/j.jtos.2025.01.008 (2025). [DOI] [PubMed] [Google Scholar]
- 39.Jaskiewicz, K. et al. The impaired wound healing process is a major factor in remodeling of the corneal epithelium in adult and adolescent patients with keratoconus. Invest. Ophthalmol. Vis. Sci.64, 22. 10.1167/iovs.64.2.22 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kourtzelis, I., Hajishengallis, G. & Chavakis, T. Phagocytosis of apoptotic cells in resolution of inflammation. Front. Immunol.11, 553. 10.3389/fimmu.2020.00553 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niederer, R. L., Perumal, D., Sherwin, T. & McGhee, C. N. J. Laser scanning in vivo confocal microscopy reveals reduced innervation and reduction in cell density in all layers of the keratoconic cornea. Invest. Ophthalmol. Vis. Sci.49, 2964–2970. 10.1167/iovs.07-0968 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Lasagni Vitar, R. M., Fonteyne, P., Chaabane, L., Rama, P. & Ferrari, G. A hypothalamic-controlled neural reflex promotes corneal inflammation. Invest. Ophthalmol. Vis. Sci.62, 21. 10.1167/iovs.62.13.21 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lasagni Vitar, R. M., Rama, P. & Ferrari, G. The two-faced effects of nerves and neuropeptides in corneal diseases. Prog Retin Eye Res.86, 100974. 10.1016/j.preteyeres.2021.100974 (2022). [DOI] [PubMed] [Google Scholar]
- 44.Dou, S. et al. Single-cell atlas of keratoconus Corneas revealed aberrant transcriptional signatures and implicated mechanical stretch as a trigger for keratoconus pathogenesis. Cell. Discov.8, 66. 10.1038/s41421-022-00397-z (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Froghi, S. et al. New insights on the role of TRP channels in calcium signalling and immunomodulation: Review of pathways and implications for clinical practice. Clin. Rev. Allergy Immunol.60, 271–292. 10.1007/s12016-020-08824-3 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilcox, N. C. et al. Interactions between skin-resident dendritic and Langerhans cells and pain-sensing neurons. J. Allergy Clin. Immunol.154, 11–19. 10.1016/j.jaci.2024.03.006 (2024). [DOI] [PubMed] [Google Scholar]
- 47.Guarino, B. D., Paruchuri, S. & Thodeti, C. K. The role of TRPV4 channels in ocular function and pathologies. Exp. Eye Res.201, 108257. 10.1016/j.exer.2020.108257 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alavi, M. S., Soheili, V. & Roohbakhsh, A. The role of transient receptor potential (TRP) channels in phagocytosis: A comprehensive review. Eur. J. Pharmacol.964, 176302. 10.1016/j.ejphar.2023.176302 (2024). [DOI] [PubMed] [Google Scholar]
- 49.Li, Y., Dai, Y., Xu, J. & Zhang, J. Transcriptomic analysis of keratoconus in Han Chinese patients: Insights into differential gene expression and ethnic-specific patterns. Exp. Eye Res.248, 110118. 10.1016/j.exer.2024.110118 (2024). [DOI] [PubMed] [Google Scholar]
- 50.Thomasy, S. M., Leonard, B. C., Greiner, M. A., Skeie, J. M. & Raghunathan, V. K. Squishy matters—Corneal mechanobiology in health and disease. Prog Retin Eye Res.99, 101234. 10.1016/j.preteyeres.2023.101234 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pintus, F., Floris, G. & Rufini, A. Nutrient availability links mitochondria, apoptosis, and obesity. Aging (Albany N.Y.)4, 734–741 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baysarowich, R. et al. Socioeconomic status and brain development: Insights and theoretical perspectives on deficit, adaptation, and resilience. Curr. Opin. Behav. Sci.63, 101502. 10.1016/j.cobeha.2025.101502 (2025). [Google Scholar]
- 53.Hackman, D. A., Farah, M. J. & Meaney, M. J. Socioeconomic status and the brain: Mechanistic insights from human and animal research. Nat. Rev. Neurosci.11, 651–659. 10.1038/nrn2897 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Muscatell, K. A., Brosso, S. N. & Humphreys, K. L. Socioeconomic status and inflammation: A meta-analysis. Mol. Psychiatry25, 2189–2199. 10.1038/s41380-018-0259-2 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Credendino, S. C., Neumayer, C. & Cantone, I. Genetics and epigenetics of sex bias: Insights from human cancer and autoimmunity. Trends Genet.36, 650–663. 10.1016/j.tig.2020.06.016 (2020). [DOI] [PubMed] [Google Scholar]
- 56.Tiberi, J. et al. Sex differences in antioxidant defence and the regulation of redox homeostasis in physiology and pathology. Mech. Ageing Dev.211, 111802. 10.1016/j.mad.2023.111802 (2023). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available in the National Center for Biotechnology Information Sequence Read Archive repository under BioProject accession number PRJNA1303973.
The code that support the findings of this study are available from the corresponding author upon reasonable request.