Graphical abstract
Keywords: Crayfish, Ozone micro-nano bubbles washing, Ultrasound washing, Well-being, Food quality
Abstract
Maintaining the well-being of living aquatic products before processing is crucial to guarantee best food quality. This study investigated the different effects of ozone micro-nano bubbles washing (OMW) and ultrasound washing on the well-being of living crayfish, one of the most economically valuable freshwater aquatic products in China. It has been found that OMW was a gentler and more effective alternative to traditional ultrasonic washing (UW) that reduced physical damage, thus leading to enhanced living vitality, increased intestinal content evacuation rate, and improved surface cleanliness of crayfish before processed as aquatic food. These improvements were attributed to the milder physical force generated by bubbles contraction and collapse than the cavitation and mechanical effects of UW. In addition, the strong cavitation, mechanical, and thermal effects of UW impaired the antioxidant system of crayfish, leading to the severe hepatopancreas damage as indicated by the substantially elevated activities of alanine aminotransferase and aspartate aminotransferase. Moreover, OMW significantly lowered the lactate dehydrogenase activity and reduced lactic acid accumulation due to decreased oxidative stress, thereby preventing acidosis in crayfish. These findings demonstrated that OMW was a milder yet more effective approach to improve the well-being of living aquatic products, thus maintaining their vitality and quality prior to processing into foods, suggesting its great potential as a pretreatment technology for applications in the aquatic food industry.
1. Introduction
The red swamp crayfish (Procambarus clarkii) was introduced into China in the late 1930s and has now become one of the most economically valuable freshwater species in Chinese aquaculture [1]. In 2023, China’s total crayfish production reached 2.89 million tons [2]. It is currently the third largest farmed crustacean species worldwide [3]. Owing to its excellent nutritional value and desirable taste, crayfish is becoming increasingly popular among consumers. The production of crayfish processed products has increased year by year [4], and China’s annual consumption has increased from 1.64 million tons to 2.39 million tons over the past three years [5]. However, the primary habitats for crayfish are wild ponds and rice fields, where they are exposed to silt, parasites, and microorganisms, which pose significant food safety risks [6]. Despite the growing market and nutritional significance of crayfish, there are problems with scientific research on their processing and quality control. Bean et al. [7] confirmed that crayfish are one of the important vectors of foodborne illness and that people’s Vibrio infections have been linked to the consumption of cooked crayfish. Dong et al. [8] also reported that Vibrio parahaemolyticus isolated from live crayfish, can cause diseases such as gastroenteritis, wound infection, and septicemia, which not only pose serious health risks to the consumers, but also lead to huge economic losses. Foodborne illnesses have become one of the most prevalent public health hazards worldwide [9].
One of the critical challenges in crayfish processing is the difficulty in cleaning, especially removing microorganisms on the body surface and gut contents of crayfish which can significantly affect their appearance and freshness. Therefore, it is particularly necessary to carefully clean and pretreat crayfish prior to catering and industrial processing. Currently, the main pretreatment method for crayfish in restaurants and small factories is manual brushing. However, the method is labor-intensive, time-consuming, and inefficient. To increase the efficiency of decontamination, chemical disinfectants, such as chlorine, have been widely used in recent decades, though they may leave chemical residues and produce carcinogenic byproducts [10]. The growing customer demand for a healthy diet is prompting manufacturers to investigate alternative solutions to preserve the quality of crayfish in a safe, effective, and ecologically responsible manner. Common non-thermal physical food processing technologies such as cold plasma, irradiation, high pressure, ozonation, ultrasound, ultraviolet light, pulsed light, and pulsed electric fields technologies are often used to clean food [11]. However, each technology has limitations. For instance, cold plasma has limited efficacy in decontaminating the inner layers of foodstuffs and is generally not suitable for high-fat foods [12]; irradiation may cause potential mass changes, such as lipid oxidation, surface discoloration, and development of off-flavors in food products [11]; high pressure treatment is not effective for products with bubbles or particles [13]; ultraviolet and pulsed light have poor penetration in opaque or complex foods [14]; pulsed electric field are mainly applied to liquid or semi-liquid food [15]. The drawbacks of existing technologies restrict their practical implementation and necessitate the development of other technologies.
Ultrasonic washing (UW) is regarded as a safe, simple, effective, and economical method that has been widely used for washing and sterilization of crayfish [16,17], and its high-power sound waves can produce cavitation in aqueous solutions and generate numerous bubbles around the products. When these bubbles burst, they apply a mechanical force to the pollutants and attachments on the product surface, facilitating cleaning and the inhibition of microorganisms within the product [18]. The versatility and efficiency of UW have made it a valuable tool in various industries. For instance, it has been successfully applied in the cleaning of cockle shells [19] and the preservation of baby cabbage [20]. Furthermore, ultrasound was also widely used in the sterilization and quality control of crayfish [17]. Despite its advantages, UW has certain limitations. Some studies suggested that, bacteria are commonly dislodged by the action of ultrasonic waves in water, rather than being lethally destroyed. UW treatment might not entirely eliminate microbial contamination within food, and the elevated water temperatures resulting from its application can contribute to additional decline in food quality, such as lipid oxidation, alterations in color and texture, along with undesirable odors [21]. Li et al. [18] also found that excessive ultrasonic power reduced water-holding capacity and caused structural alterations in myofibrillar proteins of frozen tuna. Given these constraints, UW may no longer be an ideal approach.
Ozone micro-nano bubbles washing (OMW) technology has been adopted as a novel disinfection technique for the pretreatment of food. OMW outperforms traditional large-bubbles treatments in unique aspects such as size distribution, efficiency of mass transfer, interface properties, and chemical characteristics [22]. OMW treatment generates a significant quantity of hydroxyl radicals (·OH), capable of degrading organic pollutants through sophisticated oxidation processes [23,24]. Additionally, these radicals can exert lethal effects on foodborne microorganisms by disrupting their cell membranes [25]. Because of its unique properties, OMW has become a new type of food pretreatment and disinfection technology. Research on OMW treatment has explored its efficacy in applications such as descaling ceramic membranes [26], food quality improvement [[27], [28], [29]] and wastewater management [22]. OMW has been validated for its ability to inhibit microbial growth and preserve the quality of fresh food, yet its potential to improve the quality of crayfish is not well understood. This study, therefore, investigated the influence of OMW technology on the pretreatment of crayfish through physiological and metabolic responses, with UW treatment as a comparative analysis. The aim is to position OMW as a potential safe and effective pretreatment technology for live crayfish, providing theoretical foundations and technical support for the development of the food processing industry and the enhancement of economic benefits.
2. Materials and methods
2.1. Animal materials and washing equipment
The healthy experimental crayfish were collected from the local Seafood Market (Zhoushan, China), with an average weight of 16.2 ± 4.1 g, and intact appendages. They were transported to the laboratory within an hour using ice-packs. Besides, the purchased crayfish were not pretreated in any way. The UW was performed using the ultrasound machine with the power of 480 W (Model F-080SD, Fuyang Technology Co., Ltd., Shenzhen, China). The OMW was carried out using the Uish food washing machine with the power of 210 W (Model C02, Zhejiang Uish Environmental Technology Co., Ltd., Ningbo, China). The micro-nano bubbles used in this research were produced by a spiral liquid flow-type bubbles generator and the diameters of the ozone micro-nano bubbles mainly ranged from 32 nm to 460 nm, and the Sauter mean diameter was 247 ± 9 nm [30].
2.2. Sample collection and treatments
In the experiment, the crayfish were randomly divided into 5 groups, consistent with previous studies, with 3 replicates per group and 30 crayfish per replicate, for a total of 90 crayfish per group [31]. The design of the washing time at 5 min and 10 min was based on the findings that shorter than 5 min generally led to unsatisfactory cleaning and sterilization effects as shown in Table S1, Fig. S1 and Fig. S2. For all samples, one group was untreated and labelled as CON; two groups were ultrasonically washed for 5 and 10 min, labelled as UW5 and UW10, respectively; and the remaining two groups were washed by ozone-micro bubbles for 5 and 10 min, labelled as OMW5 and OMW10, respectively. During each treatment, the temperature was maintained at room temperature (25 ± 2 °C), and the temperature of water in each treatment was (20 ± 1 °C) [32].
The collection of hemolymph in crayfish and the determination of associated enzyme activity refer to previous studies [33], and the samples were stored at −80 °C for subsequent biochemical analysis. After obtaining hemolymph samples, the hepatopancreas were isolated and prepared for histopathological examination with reference to the method [34]. In addition, intestinal tracts were isolated and collected to determine the crayfish’s intestinal contents evacuation rate.
2.3. Determination of dissolved oxygen (DO) in water
The DO concentration was continuously monitored using a DO meter (Model JPB-607A, Shanghai, China) [35].
2.4. Determination of limb damage rate (LDR) and tail flip time
The limb damage rate was defined as LDR. The number of limbs remaining in each group of crayfish after washing was denoted as b1. The LDR was then calculated using the following formula:
The tail flip time for each crayfish was measured using a stopwatch. The time was recorded 3 times for each crayfish and the mean value was calculated.
2.5. Determination of intestinal contents evacuation rate (ICER)
The intestinal contents evacuation rate was recorded as ICER. The collected intestines were rinsed with normal saline to remove mucus and pollutants from the surface. The intestines then were dried with filter paper, weighed and recorded as W1. After that, the intestinal contents were rinsed with normal saline using a syringe, dried with filter paper, weighed and recorded as W2. The specific calculation formula was as follows:
2.6. Determination of microorganisms in the crayfish abdomen
Total viable count (TVC) was carried out using the protocol outlined by Yan et al. [36], with minor adjustments. The crayfish abdomen shells were collected and accurately weighed 5.0 g, then mixed with 45 mL of sterile 0.85 % (w/v) NaCl solution and stirred for 2 min. Sterilized glass beads were added to ensure complete contact with the mixture. Then microorganisms were cultured on plate count agar (Hangwei, Hangzhou, China) at 37 °C for 48 h. The quantity of cultivated colonies was expressed as colony-forming units per milliliter of sample (CFU/mL).
2.7. Histological analysis of the hepatopancreas
For histological analysis, hepatopancreatic tissue was randomly selected from each of the five groups. The samples were rinsed with PBS buffer to remove surface blood stains and mucous membranes, then fixed in 4 % paraformaldehyde solution (Solarbio, Beijing, China) overnight. Hepatopancreas tissue samples were prepared according to Yang et al. [37], then scanned using an optical microscope (Olympus BX51, Tokyo, Japan). The magnification of the scanned image was adjusted to 20 × .
2.8. Determination of hemolymph biochemistry
Commercial biochemical kits (Nanjing Jiancheng Institute of Bioengineering, China) were used to measure the glycogen content in the crayfish tail muscle. The same methods were used to detect the total protein (TP), lactate (LD), total cholesterol (T-CHO), triglycerides (TG), glucose (GLU), total antioxidant capacity (T-AOC) and lactate dehydrogenase (LDH) activity in hemolymph. The procedures followed the manufacturer’s instructions [38]. The detection biomarkers by the same method included the following indicators: aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities; antioxidant indicators: catalase (CAT), superoxide dismutase (SOD) and glutathione reductase (GR) activities; and non-specific immunity indicators: acid phosphatase (ACP) and alkaline phosphatase (AKP) activities [39].
2.9. Statistical analysis
For each test, at least three parallel experiments were conducted and the values of each variable were expressed as the mean ± SD. Using SPSS software (Ver 17.0), One-way (ANOVA) and Duncan’s multiple range test were applied to assess the significance of differences (p < 0.05). Normality, homogeneity and independence tests were performed on all data before conducting the ANOVA.
3. Results and discussion
3.1. Effects of different washing methods on LDR and tail flip time of crayfish
For live crayfish, vitality is a key issue affecting food quality [5]. Previous studies have demonstrated the importance of crayfish limbs in competitive behaviour and their utilization of the ability to flip tails to avoid danger for survival [40]. Table 1 showed that the UW method resulted in the loss of crayfish limb, which amounted to 3.33 % and 5.67 % for UW5 and UW10 groups, respectively (p < 0.05). However, the OMW treatment did not cause limb damage in the crayfish, and the limbs remained intact. Additionally, under the influence of ultrasound, the tail flip time of the UW5 group reached 9.18 s, which was equivalent to three times longer than that of the CON group, while the UW10 group showed an inability to perform tail flips (Video S1). In contrast, the effect of the OMW on the tail flip time of crayfish was not significant. The results showed that pretreatment with OMW was superior to UW in maintaining the limb integrity and vitality of crayfish.
Table 1.
The concentration of DO in water, the LDR and tail flip time of crayfish in different washing methods.
| DO (mg/L) | LDR (%) | Tail flip time (s) | |
|---|---|---|---|
| CON | 8.82 ± 0.02b | 0c | 2.90 ± 0.26d |
| UW5 | 8.07 ± 0.13c | 3.33 ± 0.22 %b | 9.18 ± 0.55a |
| UW10 | 7.04 ± 0.05d | 5.67 ± 0.34 %a | unable tail flip |
| OMW5 | 10.90 ± 0.03a | 0c | 3.59 ± 0.56c |
| OMW10 | 11.11 ± 0.52a | 0c | 4.26 ± 0.51b |
NOTE: Different superscripts letters in the same column indicate significant differences at p < 0.05.
The primary effects of ultrasound are mechanical and cavitation effects, resulting from the forward motion of the wave in the medium [41]. High-intensity ultrasonic waves pass through a liquid medium, creating areas of alternating compression and expansion. These changes in pressure tend to produce cavitation, which leads to the creation of tiny bubbles within the medium. These bubbles often exhibit side effects by generating high temperatures and pressures during adiabatic contraction and collapse [42]. Moreover, ultrasonic waves create tensile stresses in water, resulting in negative pressure. Thermal effects are always accompanied by mechanical effects. The thermal effect of ultrasound involves the conversion of energy that occurs as the ultrasound passes through a medium. Part of its energy is converted into thermal energy through friction and heat conduction processes, leading to a localized temperature increase in the medium. The stress response of crayfish in the UW5 and UW10 treated groups was so severe that it resulted in limb mutilation, decreased vitality, and difficulty in performing tail flips (Table 1), possibly due to the influence of acoustic pressure and high temperature generated by ultrasound, which also decreased DO concentration in the UW group (Table 1).
The OMW utilizes hydraulic shear and high-speed cyclone to form shear force, which repeatedly shears and breaks up the air to mix with water thus generating a large number of ozone micro-nano bubbles [43]. In Table 1, the increase of DO in the OMW group is closely related to ozone micro-nano bubbles’ high specific surface area [44] and internal pressure [45], which considerably boost the gas dissolution efficiency in water [46]. As the ozone micro-nano bubbles rise in water and break at the surface, the gases dissolved in them decompose thermally as they collapse (Scheme 1). Ozone micro-nano bubbles collapse at the gas–liquid interface released reactive oxygen species (ROS), including ·OH. Their high stability and small size reduced crayfish limb damage and vitality loss, rendering OMW treatment less harmful than UW treatment. Study findings, based on LDR and tail-flipping time, confirmed the gentler nature of OMW, making it a more suitable preprocessing method for crayfish prior to further processing. Lim et al. [47] also found that microbubbles treatment had no effect on the survival rate and vitality of Penaeus vannamei, and promoted its growth.
Scheme 1.
The mechanism of increasing the concentration of oxygen, ozone, and hydroxyl radicals in OMW.
3.2. Effects of different washing methods on ICER and microorganisms in the crayfish abdomen
Crayfish are often held or transported for extended periods prior to processing, during which microbial proliferation can lead to spoilage and toxin production, posing health risks and threatening food safety [48]. Consequently, intestinal contents and microorganisms in crayfish could degrade the quality and even potentially lead to foodborne illness [49].
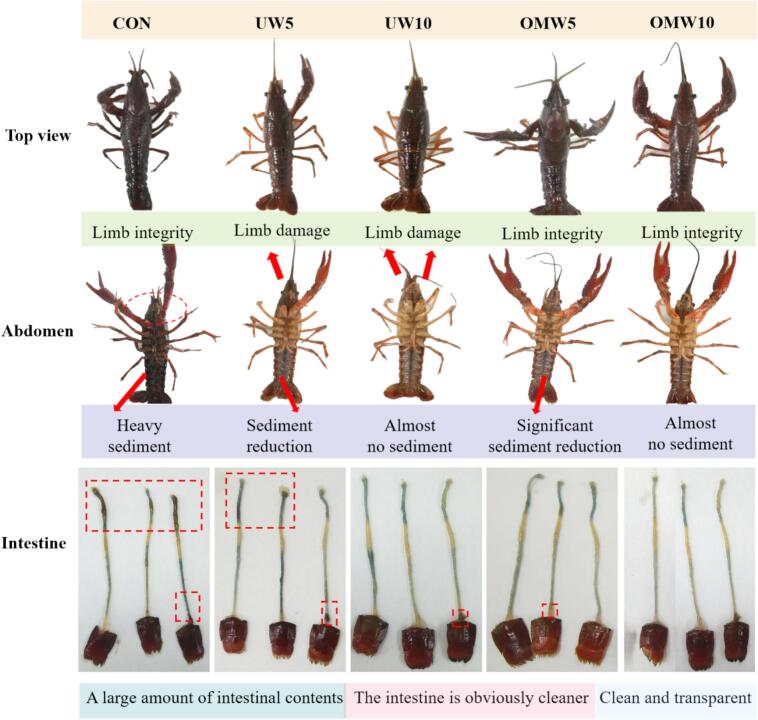
Fig. 1 revealed that the total viable count in the abdomen microorganisms of crayfish in CON and UW5 groups was notably higher than that of the other treatment groups (p < 0.05). The results also showed that UW10, OMW5 and OMW10 had better cleaning effect on the abdomen of crayfish and the difference was not significant, suggesting that OMW could achieve the sterilization effect similar to UW in half the time. Correspondingly, in Fig. 1, ICER values of crayfish treated with both UW and OMW were significantly reduced compared with CON (p < 0.05). UW5 and OMW5 treated crayfish were 21.41 % and 22.54 %, respectively, which also supported the above conclusion. Additionally, as highlighted in the red boxes in Fig. 2, the CON and UW5 groups exhibited a greater presence of intestinal content in the crayfish, further supporting the superior efficacy of the OMW in evacuating the crayfish intestine content compared to the UW.
Fig. 1.
The total viable count of microorganisms in the abdomen of crayfish (left axis) and ICER (right axis) under different washing methods. Vertical lines indicate the mean ± SD (N ≥ 3). Different letters in the same group indicate significant differences (p < 0.05).
Fig. 2.
Crayfish appearance and intestines after different washing methods.
Ultrasonic treatment’s disinfecting action is attributed to its cavitation effect, which involves the cyclical pressure changes that ultrasound induces in a medium, leading to cavitation bubbles formation [50]. The collapse of these bubbles upon pressure reduction releases energy that can irreversibly damage microbial cells by disrupting cell walls and membranes, causing cell death [51]. Additionally, ultrasound also affects microbial biofilm formation by inducing mechanical vibrations, which prevent microbial attachment to aquatic product surfaces and reduce bacterial loads [52]. In this study, the partial reduction in the number of microorganisms on the surface of crayfish in the UW group might be attributed to the aforementioned mechanisms.
Ozone’s bactericidal action is mediated through reactions with bioactive macromolecules in microbial cell membranes, altering membrane permeability and disrupting macromolecular structures. It decomposes rapidly post-treatment, leaving no residue [53]. As depicted in Scheme 1, the collapse of ozone micro-nano bubbles releases energy, generating high potential differences and cavitation, which physically remove impurities from product surfaces. The energy release from bubble rupture and gas–liquid interface disappearance is attributed to the accumulation of ions, producing a significant amount of ·OH [54]. The observed reduction in crayfish abdominal bacterial counts following OMW treatment was likely due to the potent oxidizing action of ·OH produced by ozone micro-nano bubbles. Supporting this, Liu et al. [55] identified ·OH as the primary microbial inactivation agent and Ling et al. [56] also reported significant reductions in viable bacteria and pathogens on crayfish surfaces post-ozone water treatment.
3.3. Effects of different washing methods on energy metabolism of crayfish
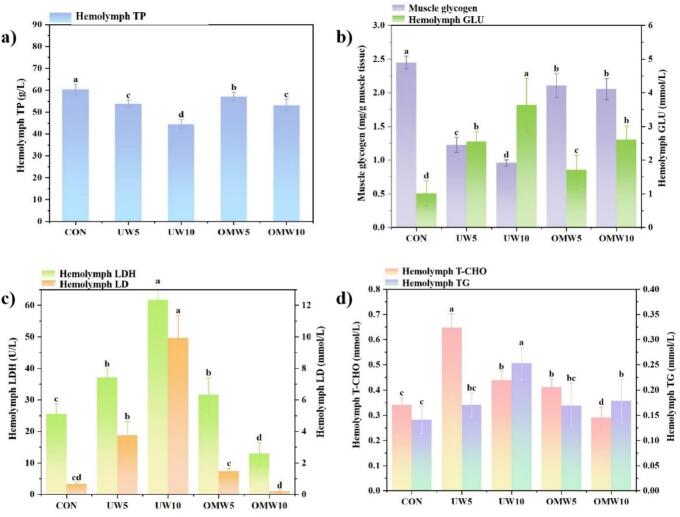
When negative environmental changes occur, crustaceans often alter their energy metabolism to adapt, generating more energy to counteract the adverse effects of the unfavorable external environment [57,58]. Therefore, the crayfish exhibited stress-induced struggling during washing, which affects their vitality and energy expenditure. Haemolymph protein levels, recognized as health indicators in crayfish [33], and proteins being a primary energy source [59], prompt this study to evaluate crayfish energy metabolism by quantifying haemolymph proteins. Fig. 3(a) showed a consistent decline in haemolymph protein content across experimental groups, with more significant reductions in the UW compared to the OMW. Notably, protein levels in the UW5 and UW10 groups decreased by 10.91 % and 26.37 %, respectively, while the decrease in the OMW group was only about half that of the UW group. This suggested lower protein consumption in OMW-treated crayfish.
Fig. 3.
Physiological indices of crayfish in different washing methods: a) Haemolymph TP; b) Muscle glycogen (left axis) and haemolymph GLU (right axis); c) Haemolymph LDH (left axis) and LD (right axis); d) Haemolymph T-CHO (left axis) and TG (right axis). Vertical lines indicate mean ± SD (N ≥ 3). Different letters in the same group indicate significant difference (p < 0.05).
The decrease in protein content may indicate energy supplementation for washing-induced exertion and stress-induced proteolysis. Moreover, proteolysis was likely a response to stress and metabolic intermediates. The decline in haemolymph protein levels might also be attributed to the disruption of protein digestion, absorption, or synthesis processes in the hepatopancreas. Similar energy metabolic responses have been observed in shrimp exposed to cypermethrin and microplastics [60]. The micro-nano bubbles, characterized by small diameter and high stability, create a milder environment for crayfish, reducing their struggle and protein consumption, thus minimizing the impact on energy metabolism. Additionally, metabolism is crucial for organisms adapting to temperature extremes [61]. In this study, UW10 increased water temperature, lowering DO (Table 1). This process temporarily excited the crayfish, shown by increased activity, significantly affecting their metabolism and raising protein consumption. Furthermore, ultrasound could alter protein structures by disrupting chemical bonds, as demonstrated by Sun et al. [62]. Therefore, UW treatment is more unfavorable.
Hemolymph GLU serves as a well-established biomarker for physiological stress in crustaceans, with Lazarevic et al. [63] have demonstrated that crayfish GLU levels respond to stressors. Fig. 3(b) presented the dynamic changes in muscle glycogen and GLU concentrations under various treatments. The UW group showed a notable decline in muscle glycogen and an approximate tripling of hemolymph GLU levels (from 1.01 to 3.63 mmol/L), while the OMW group had a less pronounced reduction in glycogen and smaller GLU fluctuations (p < 0.05). The decrease in muscle glycogen might reflect the crayfish’s need to convert muscle glycogen into glucose for energy supply during cleaning. These findings are consistent with Zhang et al. [64].
In comparison with the CON group as illustrated in Fig. 3(c), the UW10 group displayed a 16-fold surge in LD levels (0.66 to 9.93 mmol/L) and a 2-fold increase in LDH activity, indicating that the crayfish were performing anaerobic respiration under hypoxic conditions [65]. Ultrasonic treatment’s unfavorable effects elicit stress responses in crayfish, increasing oxygen consumption [66,67]. As water temperature rises, DO decreases, prompting a shift from aerobic to anaerobic metabolism in crayfish [65,68,69], thereby increasing LDH and LD activity in the UW group. Oxygen scarcity leads to LD accumulation, which can induce respiratory or metabolic acidosis, a significant threat to crayfish health. Remarkably, the crayfish in the OMW10 group displayed a significant reduction in LDH activity, reaching only 12.93 mmol/L, which was half of the CON group, while the LD activity decreased by 72.73 %, indicating improved oxygen utilization. This is attributed to OMW’s ability to enhance DO via ozone micro-nano bubbles, which facilitate LD conversion to CO2 and H2O through aerobic pathways. The inhibition of LDH activity consequently slowed down the catabolic reactions of glucose, resulting in the crayfish from the OMW10 group maintaining a higher level of GLU (2.61 mmol/L), as depicted in Fig. 3(b), consistent with previous studies [70,71].
OMW treatment utilized the high-pressure environment within the pump cavity, allowing air to rapidly dissolve in water, significantly increasing the DO concentration in the water (Table 1) [45]. Additionally, water molecules are directed to the surface of ozone micro-nano bubbles, and OH− with a negative potential is suspended in the ozone micro-nano bubbles. OH− combines with O3 to form O2, increasing the amount of DO in the water. The strong oxidant ·OH generated may influence enzyme activity. With ample DO, LD is converted into CO2 and H2O, leading to a significant decrease in LD levels in crayfish treated with OMW10.
Concurrent with glucose mobilization, lipid metabolism is dynamically regulated during stress. T-CHO and TG serve as critical biomarkers for lipid homeostasis and energy reserves [72]. In Fig. 3(d), the T-CHO levels in crayfish from the experimental groups fluctuated, peaking at 0.65 mmol/L in the UW5 group—approximately double that of the CON group—before dropping to 0.44 mmol/L after UW10 treatment, while TG content progressively increased. These fluctuations reflect stress-induced lipolysis to compensate for glycogen depletion, followed by eventual lipid exhaustion due to sustained energy demands. As cholesterol is a precursor for steroid hormone synthesis, crucial for crustacean immunity, reduced T-CHO levels in the UW10 group suggest potential immune system compromise. T-CHO also supports gut microbiota stability and intestinal health [72]. The more stable T-CHO and TG levels observed in the OMW groups indicate that OMW treatment could relieve oxidative stress and maintain metabolic balance (Fig. 3d). OMW treatment improved DO, thereby reducing reliance on anaerobic pathways and reducing lipid catabolism. This aligned with Tian et al. [73], who emphasized that sustained oxygen supply minimizes protein and lipid degradation in stressed aquatic organisms.
3.4. Effects of different washing methods on oxidative stress and immune response in crayfish
Oxidative stress, a another indicator of animal health, can lead to cellular damage and diseases when the balance between oxidants and antioxidants is disrupted [74]. Stressful conditions can trigger excessive ROS production in aquatic animals, causing severe cellular damage. Aerobic organisms, including crustaceans, employ antioxidant defense systems such as SOD, CAT, and GR to neutralize ROS, mitigate oxidative stress, and protect against or repair damage, which are integral to their immunological responses to environmental stress [75]. Widely acknowledged as trustworthy biomarkers for evaluating oxidative stress and immune response in aquatic organisms, SOD, CAT, and GR are the first line of defense against oxidative stress and are essential enzymes for shielding cells from oxidative damage and preserving cellular redox balance [76,77].
Fig. 4(a) depicted a significant increase in antioxidant enzyme activities in crayfish from the UW group, with SOD activity nearly double. SOD and CAT, key antioxidants, neutralize ROS and serve as oxidative stress biomarkers. Their increased activities indicated an activated antioxidant response to stress, with SOD converting superoxide to H2O2 and O2, which CAT then decomposes into harmless metabolites [33]. This trend in the UW group reflected a substantial stress response due to ultrasound’s three major effects. Conversely, in the OMW group, CAT activity initially increased and then decreased. Because of its strong oxidizing properties, ·OH can enter cells quickly and affect internal enzymes and cell membrane components. Initially, SOD and CAT activities in the OMW group increased slightly but decreased with extended exposure, suggesting an enhanced re-adaptation capacity. This aligned with Jomova et al. [78], who found that antioxidant enzymes were significantly elevated when organisms were stressed and that they reduced oxidative damage by catalyzing the breakdown and conversion of free radicals.
Fig. 4.
Indicators of enzyme activities in crayfish under different washing methods: a) Haemolymph CAT (left axis) and SOD (right axis); b) Haemolymph GR (left axis) and T-AOC (right axis); c) Haemolymph ACP (left axis) and AKP (right axis); d) Haemolymph ALT (left axis) and AST (right axis). Vertical lines indicate mean ± SD (N ≥ 3). Different letters in the same group indicate significant difference (p < 0.05).
Glutathione (GSH) is one of the most important intracellular antioxidants that neutralize free radicals and peroxides and protects cells from oxidative damage. GSH exists in two forms: reduced (GSH) and oxidized (GSSG). The GR enzyme works to maintain a balance between GSH and GSSG. In Fig. 4(b), the GR activity of crayfish doubled in the UW10 group over time, indicative of severe oxidative damage overwhelming endogenous GSH load. This mirrors findings by McLaughlin and Gunderson [79], who linked GR upregulation to ROS-driven lipid peroxidation in crustaceans. In the OMW group, GR activity was not significantly changed. This suggested that OMW was less damaging to crayfish. The difference could be attributed to the nature of oxidative stress. Although OMW generated significant ROS and ·OH, these are often scavenged through non-GSH mechanisms [80]. Unlike UW, which caused acute oxidative stress and temporarily depletes GSH, OMW induced milder stress that does not significantly impact GSH dynamics. This likely occurred as the ROS generated by OMW were either neutralized by other antioxidant systems or compartmentalized within cells [81], thereby diminishing their effect on GSH levels. Additionally, the lower intensity of oxidative stress in the OMW group may not trigger significant GR upregulation, as the cellular antioxidant capacity is sufficient to manage ROS levels [82], which was consistent with Alkan Uçkun and Barım Öz [83].
T-AOC is a crucial metric of the antioxidant capacity of the organism [84]. After washing, T-AOC in crayfish hemolymph increased significantly, UW5 increased nearly threefold, and OMW5 increased 45.45 % (Fig. 4b), indicating activation of the crayfish antioxidant system. This aligned with Jie et al. [85], who noted that antioxidant enzyme enhancement under hypoxia was a preparatory response to early physiological oxidative stress. The increase in sound pressure and temperature from ultrasonic waves led to reduced DO, consistent with our findings. After 10 min, T-AOC levels declined, but in OMW10 group, they returned to initial levels, possibly due to crayfish adaptive mechanisms and adequate DO and temperature facilitating enzyme activity restoration. In contrast, T-AOC in UW10 group plummeted to 0.06 mM, below the CON, suggesting an overwhelmed antioxidant system when ROS accumulation exceeded detoxification capabilities [86]. Li et al. [87] reported that high temperature could impair crayfish antioxidant systems, leading to decreased T-AOC, which was consistent with our results.
Hypoxic stress suppresses the immune system and increases the susceptibility of crustaceans to infectious diseases [88]. In Fig. 4(c), ACP activity of crayfish decreased by 18.01 % and 55.53 %, in the UW5 and UW10 groups, respectively (p<0.05). Similarly, AKP activity in crayfish was also reduced by nearly half in UW10 group. One possible explanation for the suppression of the immune system is due to the three major effects of ultrasound. The UW-induced immune damage was similar to that observed by Zhu et al. [89]. In contrast, ACP and AKP activity in the OMW10 group gradually recovered to levels similar to those in the CON group, suggesting that OMW treatment had a “re-adaptation” mechanism through adequate DO and reduced oxidation load. Studies have demonstrated that both UW and OMW treatments activate the antioxidant system and affect antioxidant enzyme activities. The UW treatment exacerbated oxidative damage in crayfish, conversely, the initiation of the “re-adaptation” mechanism following OMW treatment mitigated oxidative damage.
3.5. Effects of different washing methods on hepatopancreatic injury of crayfish
The hepatopancreas, which is central to crustacean innate immunity and immune molecule synthesis for pathogen elimination [90]. Its structural integrity is critical for maintaining immune functionality, as disorganized tissue architecture compromises cellular communication and molecular transport [37]. Therefore, hepatopancreas could be analyzed to assess immune system damage from washing stress in crayfish, as depicted in Fig. 5.
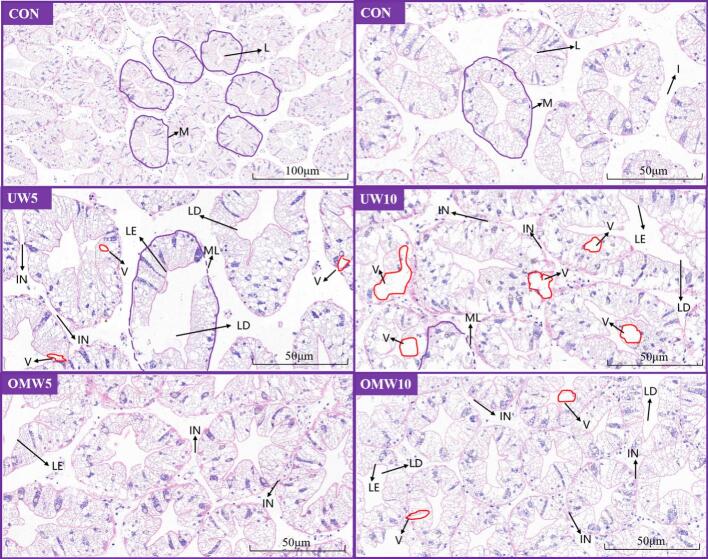
Fig. 5.
Histopathological changes of crayfish hepatopancreas exposed to washing stress. L: lumen; M: hepatocyte membrane; I: hepatocyte interstitium; V: vacuolization of hepatocytes (red circle); LE: dilatation of the hepatic tubules with a gradual expansion of the basement membrane in the inner wall of the lumen; IN: interstitial decrease of hepatocytes; LD: an increase of diameter of tubule lumen; ML: lysing of the hepatocyte membrane (Purple Coil). Bar = 50 µm.
In the CON group, crayfish hepatopancreas exhibited a fully structured, intact architecture. UW5 induced lumen dilatation, vacuolization, and reduced hepatic space, hallmarks of hepatopancreatic damage. Extended washing (UW10) exacerbated these injuries, paralleling findings in Litopenaeus vannamei under acute thermal stress where structural disorganization correlated with immunosuppression [91].
Besides, the hepatopancreas of OMW10 group showed vacuolization, but the degree was lower than that of UW5 group. An increase in the diameter of the hepatic tubules and the decrease in the interstitium were observed in the OMW5 group of crayfish, and the degree was mild and there was no hepatocyte vacuolization. As time progressed, the hepatopancreas of crayfish in the OMW10 group exhibited vacuolation, albeit to a lesser extent compared to that observed in the UW5 group. These results indicated that sound pressure, sound wave and high temperature caused by ultrasound may promote oxidative stress, which in turn impairs the hepatopancreas function of crayfish, consequently lowering antioxidant and immunological capacity. Due to the high stability and small size of ozone micro-nano bubbles, the washing environment was milder, and the crayfish in the OMW group reduced the deterioration of tissue sections. The histopathological alterations observed within the hepatopancreas of crayfish provided additional evidence supporting the observed fluctuations in hemolymph biochemical parameters and oxidative stress markers. These findings suggested that the UW treatment resulted in more pronounced lethal damage to crayfish than the OMW treatment.
Consistent with histopathological findings, hepatopancreatic biomarkers ALT and AST also respond to immune damage. In testing of hepatopancreatic performance, ALT is an indicator of hepatopancreatic cell damage, while AST is a criterion for hepatopancreatic cell necrosis. ALT and AST are mainly found in hepatopancreatic cells, and when hepatocytes are damaged or necrotic, they are released into the bloodstream, which elevates the levels of ALT and AST in the bloodstream, indicating severe liver damage [33]. Fig. 4(d) revealed a trend of increased ALT levels in crayfish hemolymph following the washing treatment, with the UW5 group increased by 47.15 %, the UW10 group increased by 93.92 %, the OMW group increased by 9.31 % and 18.19 % in the OMW5 and OMW10 groups, respectively. The ALT levels in the OMW groups were significantly lower than those in the UW groups (p<0.05). Moreover, the changes in AST levels paralleled those of ALT, and the findings were consistent with the results from the tissue sections. These results further indicated that the UW treatment caused more severe damage to the crayfish’s immune system, the OMW treatment is expected to be a potential pretreatment technique for crayfish.
Currently, OMW equipment is slightly more expensive than ultrasonic cleaning devices due to its novelty and early commercial adoption [92]. Despite the higher initial cost, OMW equipment has significantly lower operational costs, with a power consumption of 210 W compared to 480 W for ultrasonic cleaning devices in this study. This translates to reduced energy costs over time. Moreover, our study demonstrated that OMW could reduce the incidence of limb damage in crayfish, thereby enhancing their survival rates and overall quality. These improvements lead to significant economic benefits by minimizing losses and increasing marketability. Additionally, OMW achieved higher cleaning efficiency and better microbial removal, contributing to higher product value. These long-term benefits make OMW a more sustainable and cost-effective choice for food processing enterprises.
OMW showed great potential as an effective washing technique, though this study may have some limitations. Firstly, although OMW effectively eliminated common microbial contaminants and surface contamination, its efficacy against persistent pollutants such as heavy metals and pesticide residues had not yet been verified. Future work should quantify the decontamination capacity of OMW to fully assess its potential for food safety. Secondly, bubble cleaning simulation work is still in its infancy. More information about bubble cleaning is anticipated, particularly when objects have complex geometry, in order to offer more direction for real-world applications. Last but not least, our experiment was conducted on a laboratory scale, and the practicality of OMW in large-scale industrial environments still needs further verification. Further investigations into these three dimensions are expected to advance the underlying technology and broaden the practical applicability of ozone micro-nano bubbles in cleaning processes.
4. Conclusion
This study evaluated the effects of UW and OMW on crayfish washing efficiency, energy metabolism, and oxidation stress response. Through a combination of ultrasound, sound pressure and heat factors, the crayfish undergoing UW treatment experienced a fierce struggle, which caused their energy metabolism to double and muscle glycogen levels to drop, and the activity of CAT, SOD and GR in crayfish hemolymph increased significantly. However, the crayfish limbs remained intact in the OMW group, the time to flip the tail was almost unaffected, and the cleaning efficiency was significantly higher than that in the UW group. Importantly, due to the milder conditions of OMW, the effects on crayfish energy consumption and hepatopancreas damage were less. The strong oxidation by ·OH reduced LD accumulation, thereby enhancing the crayfish’s capacity for physiological re-adaptation. These results established direct correlations between crayfish physiological status and key metabolic indicators—including energy metabolism, oxidative stress, and immune response—contributing to the better understanding of the bioscience underpinned the living crayfish’s well-being improvement by OMW. As a straightforward yet effective pretreatment technology, OMW treatment could serve as a more suitable cleaning method than UW treatment prior to crayfish processing, thereby guiding its practical application in the aquatic food industry.
CRediT authorship contribution statement
Xinyan Tong: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Data curation. Lingxiang Bao: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Hanbin Lin: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Yan Chen: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Data curation, Conceptualization. Xuanju Shen: Writing – review & editing, Writing – original draft, Resources, Methodology, Investigation, Formal analysis, Data curation. Yu Xia: Writing – review & editing, Writing – original draft, Validation, Resources, Investigation, Formal analysis, Data curation. Nasra Seif Juma: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. Qinyi Zeng: Writing – review & editing, Writing – original draft, Validation, Resources, Formal analysis, Data curation. Yadong Zhao: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Investigation, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
Yadong Zhao is grateful for the financial support from National Key R&D Program of China (No. 2023YFD2401501), Science and Technology Project of Zhoushan (No. 2024C03004) and Dinghai Science and Technology Project (No. 2024C31002). Yan Chen is grateful for the financial support from Zhejiang Ocean University Talent Introduction Scientific Research Fund (No. JX6311132323).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2025.107616.
Contributor Information
Yan Chen, Email: 2022210@zjou.edu.cn.
Yadong Zhao, Email: zhaoyd@zjou.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Jin S., et al. Reproductive pattern and population dynamics of commercial red swamp crayfish (Procambarus clarkii) from China: implications for sustainable aquaculture management. PeerJ. 2019;7:24. doi: 10.7717/peerj.6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang Q., et al. Influence of different feed particle sizes on the growth performance and nutrition composition in crayfish, Procambarus clarkii Larvae. Animals-Basel. 2024;14:13. doi: 10.3390/ani14152228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xiao X., et al. Estimation of contamination level in microplastic-exposed crayfish by laser confocal micro-Raman imaging. Food Chem. 2022;397(8) doi: 10.1016/j.foodchem.2022.133844. [DOI] [PubMed] [Google Scholar]
- 4.Yan W., et al. Multi-frequency power ultrasound (MFPU) pretreatment of crayfish (Procambarus clarkii): effect on the enzymatic hydrolysis process and subsequent Maillard reaction. Ultrason. Sonochem. 2024;111(9) doi: 10.1016/j.ultsonch.2024.107140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y., et al. A comparison of eating safety and quality of live and dead freshwater crayfish (Procambarus clarkii) at different stages. Food Res. Int. 2022;159(10) doi: 10.1016/j.foodres.2022.111630. [DOI] [PubMed] [Google Scholar]
- 6.Si G., et al. Effects of an integrated rice-crayfish farming system on soil organic carbon, enzyme activity, and microbial diversity in waterlogged paddy soil. Acta Ecol. Sin. 2018;38:29–35. doi: 10.1016/j.chnaes.2018.01.005. [DOI] [Google Scholar]
- 7.Bean N.H., et al. Crayfish: a newly recognized vehicle for vibrio infections. Epidemiol. Infect. 1998;121:269–273. doi: 10.1017/S0950268898001381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dong X., et al. Characteristics of (Vibrio parahaemolyticus) isolates obtained from crayfish (Procambarus clarkii) in freshwater. Int. J. Food Microbiol. 2016;238:132–138. doi: 10.1016/j.ijfoodmicro.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Mor-Mur M., Yuste J. Emerging bacterial pathogens in meat and poultry: an overview. Food Bioprocess Technol. 2010;3:24–35. doi: 10.1007/s11947-009-0189-8. [DOI] [Google Scholar]
- 10.Chinchkar A.V., et al. Potential sanitizers and disinfectants for fresh fruits and vegetables: a comprehensive review. J. Food Process. Preserv. 2022;46:18. doi: 10.1111/jfpp.16495. [DOI] [Google Scholar]
- 11.Pankaj S.K., et al. A review of novel physical and chemical decontamination technologies for aflatoxin in food. Trends Food Sci. Tech. 2018;71:73–83. doi: 10.1016/j.tifs.2017.11.007. [DOI] [Google Scholar]
- 12.Bigi F., et al. Non-thermal techniques and the “hurdle” approach: how is food technology evolving? Trends Food Sci. Tech. 2023;132:11–39. doi: 10.1016/j.tifs.2022.12.015. [DOI] [Google Scholar]
- 13.Podolak R., et al. Factors affecting microbial inactivation during high pressure processing in juices and beverages: a review. J. Food Prot. 2020;83:1561–1575. doi: 10.4315/JFP-20-096. [DOI] [PubMed] [Google Scholar]
- 14.Mir S.A., et al. Application of new technologies in decontamination of mycotoxins in cereal grains: challenges, and perspectives. Food and Chem. Toxicol. 2021;148 doi: 10.1016/j.fct.2021.111976. [DOI] [PubMed] [Google Scholar]
- 15.Ghoshal G. Comprehensive review on pulsed electric field in food preservation: gaps in current studies for potential future research. Heliyon. 2023;9 doi: 10.1016/j.heliyon.2023.e17532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ling Y., et al. Microbial evaluation of ozone water combined with ultrasound cleaning on crayfish (Procambarus clarkii) Foods. 2022;11:16. doi: 10.3390/foods11152314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun R., et al. The combined effects of ultrasound and plasma-activated water on microbial inactivation and quality attributes of crayfish during refrigerated storage. Ultrason. Sonochem. 2023;98:14. doi: 10.1016/j.ultsonch.2023.106517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X., et al. Effect of ultrasonic thawing on the water-holding capacity, physicochemical properties and structure of frozen tuna (Thunnus tonggol) myofibrillar proteins. J. Sci. Food Agric. 2019;99:5083–5091. doi: 10.1002/jsfa.9752. [DOI] [PubMed] [Google Scholar]
- 19.Hasan M.R., et al. Efficacy of ultrasonic cleaning on cockle shells. J. Food Eng. 2023;352:10. doi: 10.1016/j.jfoodeng.2023.111523. [DOI] [Google Scholar]
- 20.Lei J., et al. The combination of non-electrolytic hypochlorite water and ultrasonic treatment on the cleaning and preservation of baby cabbage, LWT-Food. Sci. Technol. 2024;205:8. doi: 10.1016/j.lwt.2024.116528. [DOI] [Google Scholar]
- 21.Alarcon-Rojo A.D., et al. Power ultrasound in meat processing. Meat Sci. 2015;107:86–93. doi: 10.1016/j.meatsci.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 22.Zhang M., et al. Basic characteristics and application of micro-nano bubbles in water treatment, IOP Conference Series: Earth. Environ. Sci. 2020;510 doi: 10.1088/1755-1315/510/4/042050. [DOI] [Google Scholar]
- 23.Ahmed A.K.A., et al. Influences of air, oxygen, nitrogen, and carbon dioxide nanobubbles on seed germination and plant growth. J. Agric. Food Chem. 2018;66:5117–5124. doi: 10.1021/acs.jafc.8b00333. [DOI] [PubMed] [Google Scholar]
- 24.Li C., et al. Effects of ozone-microbubble treatment on the removal of residual pesticides and the adsorption mechanism of pesticides onto the apple matrix. Food Contr. 2021;120:5. doi: 10.1016/j.foodcont.2020.107548. [DOI] [Google Scholar]
- 25.Shirato M., et al. Synergistic effect of thermal energy on bactericidal action of photolysis of H2O2 in relation to acceleration of hydroxyl radical generation. Antimicrob. Agents Chemother. 2012;56:295–301. doi: 10.1128/aac.05158-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mo J., et al. Cleaning efficiency and mechanism of ozone micro-nano-bubbles on ceramic membrane fouling. Sep. Purif. Technol. 2024;331:10. doi: 10.1016/j.seppur.2023.125698. [DOI] [Google Scholar]
- 27.Shi J., et al. Ozone micro-nano bubble water preserves the quality of postharvest parsley. Food Res. Int. 2023;170:12. doi: 10.1016/j.foodres.2023.113020. [DOI] [PubMed] [Google Scholar]
- 28.Malahlela H.K., et al. Efficacy of air and oxygen micro-nano bubble waters against colletotrichum gloeosporioides and impacts on postharvest quality of 'fan retief' guava fruit. J. Food Prot. 2025;88:11. doi: 10.1016/j.jfp.2024.100437. [DOI] [PubMed] [Google Scholar]
- 29.Hou C., et al. Antibacterial efficacy and physiochemical effects of ozone microbubble water on tomato. Sustainability. 2022;14:11. doi: 10.3390/su14116549. [DOI] [Google Scholar]
- 30.Hu L., Xia Z. Application of ozone micro-nano-bubbles to groundwater remediation. J. Hazard. 2018;342:446–453. doi: 10.1016/j.jhazmat.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 31.Boštjančić L.L., et al. Dataset of the de novo assembly and annotation of the marbled crayfish and the noble crayfish hepatopancreas transcriptomes, BMC Res. Notes. 2022;15:281. doi: 10.1186/s13104-022-06137-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pian M., et al. Ozone-microbubble-washing with domestic equipment: effects on the microstructure, and lipid and protein oxidation of muscle foods. Foods. 2022;11:903. doi: 10.3390/foods11070903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao X., et al. Effect of maduramicin on crayfish (Procambius clarkii): Hematological parameters, oxidative stress, histopathological changes and stress response. Ecotoxicol. Environ. Saf. 2021;211:12. doi: 10.1016/j.ecoenv.2021.111896. [DOI] [PubMed] [Google Scholar]
- 34.Cai M., et al. Intervention of re-feeding on growth performance, fatty acid composition and oxidative stress in the muscle of red swamp crayfish (Procambarus clarkii) subjected to short-term starvation. Aquac. Res. 2021;545:13. doi: 10.1016/j.aquaculture.2021.737110. [DOI] [Google Scholar]
- 35.Han B., et al. Effects of acute hypoxic stress on physiological and hepatic metabolic responses of triploid rainbow trout (Oncorhynchus mykiss) Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.921709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan S., et al. Physicochemical and microbiological changes in postmortem crayfish (Procambarus clarkii) stored at 4 °C and 25 °C. Int. J. Food Sci. Tech. 2022;57:2992–3000. doi: 10.1111/ijfs.15620. [DOI] [Google Scholar]
- 37.Yang Y., et al. Effects of temperature on the growth parameters, hepatopancreas structures, antioxidant ability, and non-specific immunity of the crayfish. Cherax Destructor, Aquacult. Internat. 2023;31:349–365. doi: 10.1007/s10499-022-00980-x. [DOI] [Google Scholar]
- 38.Zhang X., et al. Accumulation of polyethylene microplastics induces oxidative stress, microbiome dysbiosis and immunoregulation in crayfish. Fish Shellfish Immunol. 2022;125:276–284. doi: 10.1016/j.fsi.2022.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Valipour A., et al. Dietary Lactobacillus plantarum affected on some immune parameters, air-exposure stress response, intestinal microbiota, digestive enzyme activity and performance of narrow clawed crayfish (Astacus leptodactylus, Eschscholtz) Aquac. Res. 2019;504:121–130. doi: 10.1016/j.aquaculture.2019.01.064. [DOI] [Google Scholar]
- 40.Mamdouh S., et al. Zn contamination stimulate agonistic behavior and oxidative stress of crayfishes (Procambarus clarkii) J. Trace Elem. Med Biol. 2022;69:6. doi: 10.1016/j.jtemb.2021.126895. [DOI] [PubMed] [Google Scholar]
- 41.Shi Z., et al. The effects of ultrasonic treatment on the freezing rate, physicochemical quality, and microstructure of the back muscle of grass carp (Ctenopharyngodon idella), LWT-Food. Sci. Technol. 2019;111:301–308. doi: 10.1016/j.lwt.2019.04.071. [DOI] [Google Scholar]
- 42.Khadhraoui B., et al. Review of ultrasound combinations with hybrid and innovative techniques for extraction and processing of food and natural products. Ultrason. Sonochem. 2021;76 doi: 10.1016/j.ultsonch.2021.105625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pratik A.S., James C.E. Hydroxyl ion stabilization of bulk nanobubbles resulting from microbubble shrinkage. J. Colloid Interface Sci. 2021;584:449–455. doi: 10.1016/j.jcis.2020.09.100. [DOI] [PubMed] [Google Scholar]
- 44.Li H., et al. Impact of groundwater salinity on bioremediation enhanced by micro-nano bubbles. Materials. 2013;6:3676–3687. doi: 10.3390/ma6093676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H., et al. Surface enrichment of ions leads to the stability of bulk nanobubbles. Soft Matter. 2020;16:5470–5477. doi: 10.1039/D0SM00116C. [DOI] [PubMed] [Google Scholar]
- 46.Temesgen T., et al. Micro and nanobubble technologies as a new horizon for water-treatment techniques: a review. Adv. Colloid Interface Sci. 2017;246:40–51. doi: 10.1016/j.cis.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Lim Y., et al. Effects of microbubble aeration on water quality and growth performance of Litopenaeus vannamei in biofloc system. Aquac. Eng. 2021;93:10. doi: 10.1016/j.aquaeng.2021.102159. [DOI] [Google Scholar]
- 48.Liu Y., et al. Monitoring freshness of crayfish (Prokaryophyllus clarkii) through the combination of near-infrared spectroscopy and chemometric method. J. Food Meas. Charact. 2022;16:3438–3450. doi: 10.1007/s11694-022-01451-w. [DOI] [Google Scholar]
- 49.Hernández-Pérez A., Söderhäll I. Intestinal microbiome in crayfish: its role upon growth and disease presentation. Dev. Comp. Immunol. 2023;145:10. doi: 10.1016/j.dci.2023.104703. [DOI] [PubMed] [Google Scholar]
- 50.Mu Y., et al. Combined effects of ultrasound and aqueous chlorine dioxide treatments on nitrate content during storage and postharvest storage quality of spinach (Spinacia oleracea L.) Food Chem. 2020;333:7. doi: 10.1016/j.foodchem.2020.127500. [DOI] [PubMed] [Google Scholar]
- 51.Zhang C., Xie J. Ultrasound-assisted slightly acidic electrolyzed water in aquatic product sterilization: a review. Foods. 2022;11:13. doi: 10.3390/foods11233863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang X., et al. Efficient and slurryless ultrasonic vibration assisted electrochemical mechanical polishing for 4H-SiC wafers. Ceram. Int. 2022;48:7570–7583. doi: 10.1016/j.ceramint.2021.11.301. [DOI] [Google Scholar]
- 53.Ronholm J., et al. Emerging seafood preservation techniques to extend freshness and minimize Vibrio contamination. Front. Microbiol. 2016;7 doi: 10.3389/fmicb.2016.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takahashi M., et al. Effect of microbubbles on ozonized water for photoresist removal. J. Phy. Chem. C. 2012;116:12578–12583. doi: 10.1021/jp301746g. [DOI] [Google Scholar]
- 55.Liu G., et al. Using poly (β-hydroxybutyrate-β-hydroxyvalerate) as carbon source in biofloc-systems: Nitrogen dynamics and shift of Oreochromis niloticus gut microbiota. Sci. Total Environ. 2019;694:10. doi: 10.1016/j.scitotenv.2019.133664. [DOI] [PubMed] [Google Scholar]
- 56.Ling Y., et al. Microbial evaluation of ozone water combined with ultrasound cleaning on crayfish (Procambarus clarkii) Foods. 2022;11:2314. doi: 10.3390/foods11152314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meng X., et al. Integrated physiological, transcriptome and metabolome analyses of the hepatopancreas of the female swimming crab Portunus trituberculatus under ammonia exposure. Ecotox. Environ. Safe. 2021;228:12. doi: 10.1016/j.ecoenv.2021.113026. [DOI] [PubMed] [Google Scholar]
- 58.Wang J., et al. Metabolic response in the gill of Portunus trituberculatus under short-term low salinity stress based on GC-MS technique. Front. Mar. Sci. 2022;9:14. doi: 10.3389/fmars.2022.881016. [DOI] [Google Scholar]
- 59.Guimaraes J.P.T., et al. Regulation of energy metabolism by dietary pH and protein source in diet-induced obese mice and female mice. FASEB J. 2022;36:2. doi: 10.1096/fasebj.2022.36.S1.R5584. [DOI] [Google Scholar]
- 60.Shiry N., et al. Exploring the combined interplays: Effects of cypermethrin and microplastic exposure on the survival and antioxidant physiology of Astacus leptodactylus. J. Contam. Hydrol. 2023;259:14. doi: 10.1016/j.jconhyd.2023.104257. [DOI] [PubMed] [Google Scholar]
- 61.Zimmermann S., et al. Thermal impact and the relevance of body size and activity on the oxygen consumption of a terrestrial snail, Theba pisana (Helicidae) at high ambient temperatures. Animals-Basel. 2024;14:261. doi: 10.3390/ani14020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun X., et al. Molecular and emulsifying properties of arachin and conarachin of peanut protein isolate from ultrasound-assisted extraction, LWT-Food. Sci. Technol. 2020;132:11. doi: 10.1016/j.lwt.2020.109790. [DOI] [Google Scholar]
- 63.Lazarevic J., et al. Invasive crayfish (Faxonius limosus): Meat safety, nutritional quality and sensory profile. Int. J. Environ. Res. Public Health. 2022;19:15. doi: 10.3390/ijerph192416819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang R., et al. Acute stress response in hepatopancreas of Pacific white shrimp Litopenaeus vannamei to high alkalinity. Aquacult. Rep. 2024;35 doi: 10.1016/j.aqrep.2024.101981. [DOI] [Google Scholar]
- 65.Negrete B., Jr., et al. Hypoxia-acclimation adjusts skeletal muscle anaerobic metabolism and burst swim performance in a marine fish. Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 2024;297:11. doi: 10.1016/j.cbpa.2024.111734. [DOI] [PubMed] [Google Scholar]
- 66.Sebert P., et al. Effects of a temperature increase on oxygen consumption of yellow freshwater eels exposed to high hydrostatic pressure. Exp. Physiol. 1995;80:1039–1046. doi: 10.1113/expphysiol.1995.sp003901. [DOI] [PubMed] [Google Scholar]
- 67.Meade M., et al. Metabolic physiology of the southeastern USA crayfish, Cambarus latimanus (LeConte), in response to different temperatures. Freshwater Crayfish. 2015;21:171–177. doi: 10.5869/fc.2015.v21-1.171. [DOI] [Google Scholar]
- 68.McMahon B.R., et al. Respiratory responses to long-term hypoxic stress in the crayfish Orconectes Virilis. J. Exp. Biol. 1974;60:195–206. doi: 10.1242/jeb.60.1.195. [DOI] [Google Scholar]
- 69.Zeng Q., et al. Effects of hypoxia stress on survival, antioxidant and anaerobic metabolic enzymes, and related gene expression of red swamp crayfish Procambarus clarkii. Biology. 2024;13:33. doi: 10.3390/biology13010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Verma S., et al. Pharmacologic LDH inhibition redirects intratumoral glucose uptake and improves antitumor immunity in solid tumor models. J. Clin. Invest. 2024;134 doi: 10.1172/jci177606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Green S.R., Storey K.B. Regulation of crayfish, Orconectes virilis, tail muscle lactate dehydrogenase (LDH) in response to anoxic conditions is associated with alterations in phosphorylation patterns, Comparative biochemistry and physiology. Part B, Bioc. Mol. B. 2016;202:67–74. doi: 10.1016/j.cbpb.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 72.Hou S., et al. Effects of dietary phospholipid and cholesterol levels on antioxidant capacity, nonspecial immune response and intestinal microflora of juvenile female crayfish, Procambarus clarkii. Aquacult. Rep. 2022;25:12. doi: 10.1016/j.aqrep.2022.101245. [DOI] [Google Scholar]
- 73.Tian Y., et al. Effects of intermittent fasting on growth, metabolism and stress resistance in freshwater crayfish (Procambarus clarkii) Aquacult. Rep. 2024;38 doi: 10.1016/j.aqrep.2024.102275. [DOI] [Google Scholar]
- 74.Zhang H., et al. Effects of dietary replacement of fishmeal by cottonseed meal on the growth performance, immune and antioxidant responses, and muscle quality of juvenile crayfish Procambarus clarkii. Aquacult. Rep. 2023;31:9. doi: 10.1016/j.aqrep.2023.101639. [DOI] [Google Scholar]
- 75.Duan Y., et al. Effect of desiccation on oxidative stress and antioxidant response of the black tiger shrimp Penaeus monodon. Fish Shellfish Immunol. 2016;58:10–17. doi: 10.1016/j.fsi.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 76.Stará A., et al. Effect of chronic exposure to prometryne on oxidative stress and antioxidant response in red swamp crayfish (Procambarus clarkii) Biomed Res. Int. 2014:6. doi: 10.1155/2014/680131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Miao S., et al. Effects of dietary protein level on the growth performance, feed utilization and immunity of red swamp crayfish Procambarus clarkia. Aquacult. Rep. 2020;18:7. doi: 10.1016/j.aqrep.2020.100540. [DOI] [Google Scholar]
- 78.Jomova K., et al. Several lines of antioxidant defense against oxidative stress: antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Arch. Toxicol. 2024;98:1323–1367. doi: 10.1007/s00204-024-03696-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McLaughlin Q.R., Gunderson M.P. Effects of selenium treatment on endogenous antioxidant capacity in signal crayfish (Pacifastacus leniusculus) Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2022;256:6. doi: 10.1016/j.cbpc.2022.109324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yin J., et al. The scavenging of reactive oxygen species and the potential for cell protection by functionalized fullerene materials. Biomaterials. 2009;30:611–621. doi: 10.1016/j.biomaterials.2008.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo W., et al. Reactive oxygen species: a crosslink between plant and human eukaryotic cell systems. Int. J. Mol. Sci. 2023;24:13052. doi: 10.3390/ijms241713052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borisov V.B., et al. ROS defense systems and terminal oxidases in bacteria. Antioxidants. 2021;10:839. doi: 10.3390/antiox10060839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Alkan Uçkun A., Barım Öz Ö. Acute exposure to the fungicide penconazole affects some biochemical parameters in the crayfish (Astacus leptodactylus Eschscholtz, 1823) Environ. Sci. Pollut. Res. 2020;27:35626–35637. doi: 10.1007/s11356-020-09595-2. [DOI] [PubMed] [Google Scholar]
- 84.Lu Y., et al. Effects of dietary Eucommia ulmoides leaf extract on growth, muscle composition, hepatopancreas histology, immune responses and Microcystin-LR resistance of juvenile red claw crayfish (Cherax quadricarinatus) Fishes. 2023;8:18. doi: 10.3390/fishes8010020. [DOI] [Google Scholar]
- 85.Jie Y., et al. Hypoxia-induced oxidative stress and transcriptome changes in the mud crab (Scylla paramamosain) Comp. Biochem. Physiol. C-Toxicol. Pharmacol. 2021;245:9. doi: 10.1016/j.cbpc.2021.109039. [DOI] [PubMed] [Google Scholar]
- 86.Xia X., et al. Polyvinyl chloride microplastics induce growth inhibition and oxidative stress in Cyprinus carpio var. larvae. Sci. Total Environ. 2020;716:8. doi: 10.1016/j.scitotenv.2019.136479. [DOI] [PubMed] [Google Scholar]
- 87.Li R., et al. A crayfish Ras gene is involved in the defense against bacterial infection under high temperature. Fish Shellfish Immunol. 2019;86:608–617. doi: 10.1016/j.fsi.2018.11.062. [DOI] [PubMed] [Google Scholar]
- 88.Qin F., et al. Dietary nano-selenium relieves hypoxia stress and, improves immunity and disease resistance in the chinese mitten crab (Eriocheir sinensis. Fish Shellfish Immunol. 2016;54:481–488. doi: 10.1016/j.fsi.2016.04.131. [DOI] [PubMed] [Google Scholar]
- 89.Zhu X., et al. Transcriptome analysis reveals immune and antioxidant defense mechanisms in the eriocheir japonica sinensis after exposure to ammonia. Animals-Basel. 2024;14:2981. doi: 10.3390/ani14202981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y., et al. Cadmium-induced oxidative stress, histopathology, and transcriptome changes in the hepatopancreas of freshwater crayfish (Procambarus clarkii) Sci. Total Environ. 2019;666:944–955. doi: 10.1016/j.scitotenv.2019.02.159. [DOI] [PubMed] [Google Scholar]
- 91.Han J., et al. Genomic and histopathological characteristics of Vibrio parahaemolyticus isolated from an acute hepatopancreatic necrosis disease outbreak in Pacific white shrimp (Penaeus vannamei) cultured in Korea. Aquac. Res. 2020;524 doi: 10.1016/j.aquaculture.2020.735284. [DOI] [Google Scholar]
- 92.Jin N., et al. Environment-friendly surface cleaning using micro-nano bubbles. Particuology. 2022;66:1–9. doi: 10.1016/j.partic.2021.07.008. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.