Abstract
The properties of extracellular vesicles depend on characteristics of the origin of cells. This study aimed to comprehensively evaluate a protocol for production of bone marrow mesenchymal stem cell-derived small extracellular vesicles (BM-MSC-sEVs) and investigate their effects on hydrogen peroxide (H2O2) induced cell damage to spontaneously arising retinal pigment epithelium (ARPE-19). We found that cell morphology and proliferative capacities of BM-MSCs obtained in α-MEM were higher than those cultured in DMEM, although not statistically significant. The particle yields were statistically higher when isolated by tangential flow filtration (TFF) than by ultracentrifugation (UC). Toxicity of BM-MSC-sEVs and therapeutic effects upon damaged ARPE-19 were assessed. They were found to be noncytotoxic, and to enhance ARPE-19 cell proliferation. While viability of ARPE-19 cells after H2O2 exposure was 37.86 ± 0.61%, application of sEVs (50 µg/mL) onto these cells for 24 h before or after H2O2 exposure increased viabilities to 54.60 ± 3.59% and 52.68 ± 0.49%, respectively. Flow cytometry showed significant reduction of total apoptotic cells. In conclusion, isolation of sEVs by TFF was the more effective method. Derived sEVs demonstrated capabilities as a potential therapeutic agent for retinal diseases.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-21218-9.
Keywords: Apoptosis, Bone marrow-derived mesenchymal stem cells, Proliferation, Small extracellular vesicles, Tangential flow filtration
Subject terms: Isolation, separation and purification; Mesenchymal stem cells
Introduction
Retinitis pigmentosa (RP) is an inherited retinal condition that affects one in every 3,000 individuals globally. RP is caused by genetic mutations and associated with inflammation and progressive degeneration of retinal cells1,2. The pathological mechanism of RP involves degeneration of photoreceptor cells and subsequent oxidative stress that causes dysfunction of the retinal pigment epithelium (RPE). Therefore, the regulation of oxidative stress is considered an important strategy to maintain the remaining photoreceptor cells and retinal function3.
Mesenchymal stem cells (MSCs) have emerged as a promising treatment option in ocular diseases due to their ability to modulate the immune system, regenerate tissues, and provide protective functions4,5. Bone marrow is one of the most effective sources of MSCs for cell-based therapeutic trials of degenerative retinal diseases6. The primary benefit of MSCs is the paracrine protective effect, rather than cell replacement, toward the RPE and photoreceptors. MSCs spontaneously produce growth factor, nerve growth factor, and vascular endothelial growth factor (VEGF), which act in tissue regeneration factors. MSCs also produce immunomodulatory cytokines, such as interleukin-6 (IL-6) and transforming growth factor-beta (TGF-beta), which inhibit the inflammatory reaction of the cells during disease progression7. We previously conducted a phase I clinical trial to assess the safety of intravitreal autologous MSC injection in 14 patients with advanced-stage RP. The results revealed that the treatment appeared to be safe and potentially effective. Nevertheless, several mild and one severe but manageable adverse events were observed8. To overcome these unwanted effects and still maintain the benefits of the MSC therapy, we proposed a cell-free approach using small extracellular vesicles (sEVs) from bone marrow mesenchymal stem cells (BM-MSCs).
EVs are diverse nonreplicating nanoparticles that were classified into several types, including exosomes (or at present known as sEVs), endocytic origin; 30–150 nm in size), microparticles (MPs, plasma membrane budding; 150–1000 nm), and apoptotic bodies (membrane blebbing; 800–5000 nm)9. Released EVs carry cargos, such as proteins, nucleic acids (DNA, messenger RNA, and micro RNAs), and lipids from parental cells which can be transferred to recipient cells9–11. The effects of EVs include repair of tissue damage, inhibition of inflammation, and regulation of oxidative stress12–14.
In ophthalmology, MSC-sEVs have been intensively studied. In glaucoma, an in vitro study found that the human BM-MSC-sEVs provide neuroprotective effects to injured human retinal ganglion cells and promoted their growth15. Other retinal diseases, including retinal degeneration16, retinal ischemia17,18, and uveitis19, have also been studied for the potential benefits of sEVs as an alternative therapeutic modality. Several clinical trials are evaluating their safety and efficacy: umbilical MSC-derived sEVs for the treatment of dry eye symptoms due to chronic graft-versus-host disease (NCT04213248); MSCs and MSC-sEVs to promote healing of macular holes (NCT03437759); Wharton’s jelly MSC-derived sEVs on visual function of RP patients (NCT05413148).
The manufacturing of sEVs is dependent on several factors, including characteristics of the donor(s), cell sources, culture conditions and isolation methods. These significantly impact the purity, sterility, safety, potency, and identity of sEVs20,21. Previous studies have developed culture systems, including types of media, growth factors and sources of serum supplement, culture system (two-dimensional (2D), 3D cell cultures) to support proliferation and differentiation potency of MSCs22–25. Moreover, several methods for sEV isolation have been developed to increase production yield and purity26–28. Although MSC-sEVs are generally considered relatively safe and less toxic than whole cells29, understanding of the toxicity of MSC-sEVs for retinal toxicity has not been fully elucidated. Due to the pathogenesis of RPE dysfunction, ARPE-19 cells are commonly used to assess substances for potential effects and toxicity to the retina30,31. For the first step of our assessment of sEVs as a potential therapeutic, we sought to optimize the protocols for BM-MSC culture and sEV isolation under good manufacturing practice (GMP)-compliant production, while evaluating their functional activity and toxicity on ARPE-19 cells. Here we present the results of these initial studies.
Results
Identification of bone marrow-derived mesenchymal stem cells
Bone marrow-derived mesenchymal stem cells (BM-MSCs) were obtained from the bone marrow of five donor subjects, with a mean volume of 19.60 ± 0.77 mL/person (Supplementary Table 1). All bone marrow samples were successfully differentiated into BM-MSCs, with an average of 19.17 ± 8.95 × 106 cells per sample. These cells were cultured under GMP conditions in a xeno-free culture medium. BM-MSCs demonstrated fibroblast-like morphology upon adhering to culture flasks (Supplementary Fig. 1 A). Cell viability after expansion was 98.86 ± 0.70%. Flow cytometric analysis revealed that these cells expressed high levels of CD73, CD90, and CD105 and lacked CD34, CD45, and HLA-DR (Supplementary Fig. 1B). Additionally, these cells differentiated into adipocytes, osteocytes, and chondrocytes (Supplementary Fig. 1 C). Microbiological assays revealed no bacteria, fungi nor Mycoplasma spp. The endotoxin levels were below critical limits (< 0.25 EU/mL) (Supplementary Table 2).
Effect of culture media on BM-MSCs growth and proliferative capacity
To assess the comparative ability of BM-MSCs to grow in two different culture media, BM-MSCs were cultured up to passage 6 in Dulbecco’s Modified Eagle Medium (DMEM) and Alpha Minimum Essential Medium (α-MEM) both supplemented with 10% human platelet lysate (hPL). The microscopic morphology of BM-MSCs on day 5 displayed the normal shape of fibroblasts in both media. By passage 6, the number of adherent cells on day 5 decreased in both media (Fig. 1A). Next, cell population doubling time (CPDT) analysis of the cultivated cells from each passage showed that CPDT extended from 1.90 ± 0.45 to 2.25 ± 0.46 days in the DMEM and from 1.85 ± 0.36 to 1.99 ± 0.55 days in the α-MEM from passage 3 to 6, respectively, leading to an increasing time required to achieve 90% confluency (Fig. 1B-C). Finally, the expansion ratio of BM-MSCs cultured in α-MEM was higher than those cultured in DMEM (Fig. 1D). However, the CPDT, time to confluence, and expansion ratio of BM-MSCs in DMEM and α-MEM were not significantly different (p-value > 0.05).
Fig. 1.
Cultivation and propagation of BM-MSCs in DMEM and α-MEM both supplemented with 10%hPL. (A) Morphology of BM-MSCs at passage 3 through 6, cultured in DMEM (upper panels) or α-MEM (lower panels). BM-MSCs were plated at 3,000 cells/cm2 in T75 culture flasks. The images were captured on day 5. Scale bars indicate 100 μm. (B) Cell population doubling time (CPDT). (C) Culture time to achieve 90% confluency of each passage. (D) Expansion ratio of BM-MSCs on day 5. P3, N = 4; P4-6, N = 5. ns denotes not significant. DMEM, Dulbecco’s modified Eagle’s medium; α-MEM, Minimum essential medium-alpha modification; P, Passage.
Effect of culture media on the characteristics of bone marrow mesenchymal stem cell-derived small extracellular vesicles
To compare the characteristics of BM-MSC-sEVs in each passage, the BM-MSC-sEVs were isolated from conditioned medium (CM) of each passage and each individual subject by UC and analyzed by nanoparticles tracking analysis (NTA), transmission electron microscopy (TEM), and Western blotting. Size and number of sEV particles per volume were determined by NTA. Mean size distribution of particles isolated from BM-MSCs cultured in DMEM and α-MEM were 114.16 ± 14.82 and 107.58 ± 24.64 nm, respectively (Fig. 2A). Average yields of particles/cell were 3,751.09 ± 2,058.51 and 4,318.72 ± 2,110.22 in DMEM and α-MEM, respectively (Fig. 2B). No differences were observed in the size distribution nor the yield of particles/cell among passages in either culture medium. Isolated particles examined by TEM exhibited a cup-shaped morphology (Fig. 2C-D). Western blotting confirmed the presence of CD9, CD63, and TSG101 (Fig. 2E). The whole western blot images of common sEV markers and calnexin were presented in Supplementary Fig. 2. These results indicated that the isolated particles were sEVs32. Taken together, α-MEM was selected as the medium for subsequent experiments to optimize cell proliferation and yield of BM-MSC-sEVs.
Fig. 2.
Effect of culture medium on BM-MSC-sEV secretion from individual subject. Particle size and concentration were measured by NTA. (A) Mean size of particles isolated from CM at passages 3 through 6 in DMEM and α-MEM. (B) Particle concentration. (C-D) TEM images of isolated particles cultured in DMEM (C) or α-MEM (D). The scale bars represent 200 nm. (E) Western blot assay of sEV common markers (CD9, CD63, and TSG101) and calnexin; 10 µg of total protein was loaded in each lane. The full-length of western blots were presented in Supplementary Fig. 2. Passage 3, N = 4; passages 4–6, N = 5. ns denotes not significant. DMEM, Dulbecco’s modified Eagle’s medium; α-MEM, Minimum essential medium-alpha modification; NTA, nanoparticle tracking analysis; P, Passage; sEVs, small extracellular vesicles; TEM, transmission electron microscope.
To investigate the interindividual effect of BM-MSCs on sEV secretion, BM-MSCs were cultured in α-MEM. BM-MSC-sEVs of each individual were isolated from pooled CM (passages 4 through 6). The effects are shown in Supplementary Fig. 3 and Supplementary Table 3. Size and number of sEV particles were analyzed by NTA. No differences were observed in particle size which ranged from 105 to 131 nm (Supplementary Fig. 3 A). In contrast, particle concentrations of BM-MSC-sEVs from donors 1 and 2 (BM-MSC01 and BM-MSC02) were significantly higher than those from donors 3 and 5 (BM-MSC03 and BM-MSC05) (Supplementary Fig. 3B). From these results, there was no inter-individual variability in size nor particle concentration of BM-MSC-sEVs. Therefore, BM-MSCs from donors 1, 2 and 4 were selected for comparison of BM-MSC-sEVs isolation methods in the next experiment.
Comparison of BM-MSC-sEVs isolation by ultracentrifugation and tangential flow filtration system
UC is the classical method used for EV isolation and purification33. However, a protocol for large-scale isolation of BM-MSC-sEVs using TFF has recently been established33–35. To compare the yield and quality of BM-MSC-sEVs isolated by the two methods, BM-MSC-sEVs were isolated from equal volumes (500 mL) of clear pooled CM from BM-MSCs passage 4 through 6 (donor 1, 2, and 4) using either the UC or TFF method with two different membrane pore sizes (TFF300 and TFF500 kDa). The average size distribution of BM-MSC-sEVs isolated by UC, TFF300, and TFF500 were 128 ± 4.58, 118.00 ± 11.53, and 117.67 ± 6.81 nm, respectively. No differences were observed in the size distribution of BM-MSC-sEVs (Fig. 3A), while the particle concentration and total protein of BM-MSC-sEVs isolated by TFF were significantly higher than those isolated by UC. No significant differences were found in BM-MSC-sEVs isolated by TFF based on different membrane pore size. TFF with the 300 kDa membrane produced larger yields of BM-MSC-sEVs (2.03 × 1012 ± 0.59 × 1011 particles) than TFF500 (1.41 × 1012 ± 0.27 × 1011 particles). The yields of BM-MSC-sEVs isolated by UC (6.53 × 109 ± 0.13 × 109 particles) were significantly lower than by the TFF system (Fig. 3B). Next, total protein was determined by BCA assay. BM-MSC-sEVs isolated by TFF300 (18.74 ± 3.90 µg) showed higher amount of protein compared to both TFF500 (17.62 ± 0.43 µg) and UC (1.30 ± 0.05 µg) (Fig. 3C). Purity of BM-MSC-sEVs, as determined by the ratio of total particles per microgram of protein, showed that the purity of BM-MSC-sEVs isolated by TFF300 (1.10 × 1011 ± 1.10 × 1010 particles/µg) was significantly higher than by both TFF500 (7.80 × 1010 ± 8.70 × 109 particles/µg) and UC (5.8 × 1010 ± 2.00 × 1010 particles/µg). No significant differences were found between BM-MSC-sEVs isolated by TFF500 and UC (Fig. 3D). These findings indicate that TFF300 improved the yield and purity of BM-MSC-sEVs. BM-MSC-sEV protein markers were analyzed by western blotting; CD9, CD63, and TSG101 were all detected in BM-MSC-sEVs isolated by each of the three methods. Calnexin, an endoplasmic reticulum marker, was detected only in the cell lysate, but not in any of the UC and TFF-isolated samples (Fig. 3E). The whole western blot images of common sEV markers (presented in Supplementary Fig. 4) suggested a lack of substantial contaminating cell debris in the UC and TFF-isolated samples. TEM images of BM-MSC-sEVs isolated using any of the three methods revealed a cup-shaped structure (Fig. 3F). The TFF system was comprised of three steps: concentration, diafiltration and recovery. In this experiment, two different membrane pore sizes were tested to determine the recovery yield and quality of the final BM-MSC-sEVs. TEM images demonstrated that a large number of proteins, visible as amorphous structures, were present in the filtered CM. After the diafiltration step of the final BM-MSC-sEV product, typical “cup-shaped” vesicle structures were detected (Fig. 3F). Sterility testing of BM-MSC-sEVs was negative for microbial contamination, endotoxins and mycoplasma (Supplementary Table 4). These findings indicate that a 300 kDa membrane was the most appropriate choice for the isolation of BM-MSC-sEVs.
Fig. 3.
Characterization of BM-MSC-sEVs isolated from CM using ultracentrifugation or tangential flow filtration. BM-MSC-sEVs were isolated from equal volumes (500 mL) of clear pooled CM from BM-MSCs at passage 4 through 6 (BM-MSC01, BM-MSC02, and BM-MSC04) using either the UC or TFF method with two different membrane pore sizes (TFF300 orTFF500 kDa). (A) Mean size distribution and (B) total particle number of BM-MSC-sEVs isolated by UC or TFF using NTA. (C) Total protein determined by BCA protein assay. (D) Purity of isolated particles expressed as particles per microgram of protein. (E) Western blot analysis of BM-MSC-sEVs using CD9, CD63, TSG 101, and calnexin. The full-length of Western blots are presented in Supplementary Fig. 4. (F) Transmission electron microscopy of culture medium, after diafiltration step, and BM-MSC-sEVs final product obtained by UC or TFF system. Data presented as mean ± SD. N = 3 per group. Data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. Statistically significant at * p < 0.05, ** p < 0.01 and *** p < 0.001. ns denotes not significant. BCA, Bicinchoninic acid; NTA, nanoparticle tracking analysis; sEVs, small extracellular vesicles; TFF, tangential flow filtration; UC, ultracentrifugation.
Internalization of BM-MSC-sEVs into ARPE-19 cells
BM-MSC-sEVs provide a promising approach for the treatment of various diseases. With RP in mind, we investigated the internalization of BM-MSC-sEVs into ARPE-19 cells. To exclude nonspecific fluorescent staining, a mock control containing vesicle-free phosphate-buffered saline (PBS) was included in the staining procedure (Fig. 4A). BM-MSC-sEVs isolated by UC or TFF were labelled with PKH26 and incubated with ARPE-19 cells. After 16 h of incubation, the uptake of BM-MSC-sEVs by ARPE-19 cells was observed in the cytoplasm and around the nuclei of the cells (Fig. 4B-D). At the same inbubation period (16 h), ARPE-19 cells incubated with BM-MSC-sEVs showed significantly higher red fluorescence than the control group (PBS), suggesting that BM-MSC-sEVs were taken up by ARPE-19 cells. The quantification of BM-MSC-sEVs internalization showed that BM-MSC-sEVs isolated by TFF300 was significantly higher than those incubated with BM-MSC-sEVs by TFF500 and UC, respectively (Fig. 4E). To verify if BM-MSC-sEVs could be taken up by ARPE-19 cells, time-lapse monitoring of living cells showed that the uptake of sEVs into ARPE-19 cells increased in a time-dependent manner (Supplementary Video 1). These results indicate that BM-MSC-sEVs were internalized into ARPE-19 cells.
Fig. 4.
Fluorescence-labeled BM-MSC-sEVs and internalization of BM-MSC-sEVs into ARPE-19 cells. BM-MSC-sEVs from α-MEM medium, isolated by UC or TFF (50 µg/mL), were labelled with PKH26 and co-incubated with ARPE-19 cells (15,000 cells/well) for 16 h. Then, cells were stained with Phalloidin and DAPI. (A-D) Fluorescence images of BM-MSC-sEVs isolated by UC (B), TFF300 (C), and TFF500 (D) were captured using a confocal microscope. A mock control using PBS was included in the entire staining procedure (A). Blue: Nucleus stained with DAPI. Red: PKH26-labeled BM-MSC-sEVs. Green: F-actin stained with Phalloidin. Scale bars, 100 μm. (E) Quantification of the mean intensity of the fluorescent signals after treatment with BM-MSCs-sEVs using Image J. Data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. Statistically significant at * p < 0.05, ** p < 0.01 and *** p < 0.001. ARPE-19, spontaneously arising retinal pigment epithelium; BM-MSC-sEVs, bone marrow mesenchymal stem cells-derived small extracellular vesicles; DAPI, 4’,6-diamidino-2-phenylindole; α-MEM, Minimum essential medium-alpha modification; PBS, phosphate buffered saline.
BM-MSC-sEVs have potential therapeutic effects and protective effects on ARPE-19 cells
To determine the optimal concentration of H2O2 for inducing ARPE-19 cell damage, ARPE-19 cells were treated with various concentrations of H2O2 (100, 250, 300, 350, 400, 500, 750 or 1,000 µM) for 24 h. MTS assay was used to assess cell viability. The results showed that increasing the concentration of H2O2 decreased cell viability of ARPE-19 in a dose-dependent manner. The concentration of H2O2 at 350 µM reduced cell viability by 50% (Supplementary Fig. 5 A). To evaluate potential cytotoxicity by BM-MSC-sEVs, ARPE-19 cells were incubated with 25, 50, or100 µg/mL of BM-MSC-sEVs for 24 h. The results showed that BM-MSC-sEVs treatment alone (at different concentrations) did not decrease ARPE-19 cell viability, and there were no significant differences among various concentrations of BM-MSC-sEVs from different passages, nor of BM-MSC-sEVs isolated from different culture media (Supplementary Fig. 5B-C). Additionally, cell viability of ARPE-19 cells after exposure to BM-MSC-sEVs-UC, -TFF300, or -TFF500 were 104.50 ± 1.24%, 106.20 ± 2.91%, and 102.10 ± 1.21%, respectively (Supplementary Fig. 5D). There were no significant differences in cell viability post-incubation of ARPE-19 cells. Therefore, we considered that a suitable concentration for inducing cell damage was 350 µM of H2O2; 50 µg/mL of BM-MSC-sEVs-TFF300 was selected for subsequent experiments.
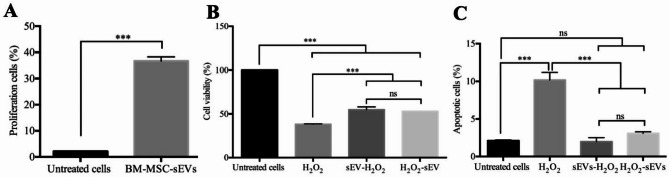
To investigate the effect of BM-MSC-sEVs on proliferation of ARPE-19 cells, a CFSE proliferation test and flow cytometry were used to assess the effects of BM-MSC-sEVs on ARPE-19 cells. The results showed the presence of two populations of ARPE-19 cells based on CFSE content, those with low CFSE were proliferating cells while those with high CFSE were non-proliferating cells (Supplementary Fig. 6 A). BM-MSC-sEVs significantly enhanced proliferation of ARPE-19 cells (36.70 ± 1.59%) compared to the untreated controls (2.16 ± 0.02%) (Fig. 5A). In order to investigate whether BM-MSC-sEVs treatment could protect ARPE-19 cells from H2O2-induced cell damage, the cells were pretreated with BM-MSC-sEVs for 24 h prior to exposure to H2O2 (for another 24 h). Cell viability of ARPE-19 cells exposed to H2O2 alone was 38.17 ± 0.43% (Fig. 5B). Interestingly, pre-treatment with BM-MSC-sEVs resulted in cell viability of 54.80 ± 3.22%, which was significantly higher than the non-treated group (p-value < 0.001). These results indicated that BM-MSC-sEVs exerted a protective effect on ARPE-19 cells. To determine the curative effect of BM-MSC-sEVs, ARPE-19 cells were exposed to H2O2 for 24 h to allow an induction of cell damage, followed by treatment with BM-MSC-sEVs for another 24 h (H2O2-sEVs). The resulting cell viability was 52.68 ± 0.49%, which indicated that BM-MSC-sEVs could interrupt or revert the H2O2 damage process in ARPE-19 cells. These results suggested that BM-MSC-sEVs had potential protective and therapeutic effects on the H2O2-induced cell damage of ARPE-19 cells. Additionally, the effect of sEVs on H2O2-induced apoptosis was observed. ARPE-19 cells were pretreated with BM-MSC-sEVs before H2O2 exposure for 24 h (sEVs-H2O2), or H2O2-sEVs. Flow cytometry was performed to determine the percentage of apoptotic cells on day 3. The results showed that total apoptotic cells of ARPE-19 were significantly reduced when BM-MSC-sEVs were added to culture conditions either before or after H2O2 challenge, compared with the H2O2 alone group (Fig. 5C, Supplementary Fig. 6B). These results suggested that BM-MSC-sEVs reduced apoptosis of ARPE-19 cells and promoted cell proliferation.
Fig. 5.
Effects of BM-MSC-sEVs on proliferation and apoptosis of ARPE-19 cells. (A) BM-MSC-sEVs were isolated from pooled CM of BM-MSCs passage 4 through 6 (BM-MSC01, BM-MSC02, and BM-MSC04) by TFF method using 300 kDa membrane cut-off cartridges. APRE-19 cells incubated with 50 µg/mL of BM-MSC-sEVs. Cell proliferation was measured using 0.5 µM carboxyfluorescein diacetate succinimidyl ester (CFSE) staining for 30 min, followed by washing and seeding in 24 well plates overnight. After treatment for 3 days, cells were harvested and quantitated by flow cytometry. From each sample, 10,000 events were acquired. Unstained cells were used as controls to differentiate between CFSE signal and background noise. Bar chart shows the percentage of divided cells when cultured in the absence or presence of BM-MSC-sEVs. (B) ARPE-19 cells were challenged with 350 µM H2O2 for 24 h to induce cell damage. The effects of BM-MSC-sEVs were evaluated in ARPE-19 cells pre-treated with BM-MSC-sEVs for 24 h before H2O2 exposure (sEVs-H2O2) for another 24 h, and cells exposed to H2O2 for 24 h followed by BM-MSC-sEVs treatment for another 24 h (H2O2-sEVs). Cell viability was measured by MTS assay. (C) Apoptotic cells were quantified on day 3 by annexin V and 7AADs using flow cytometry. Percentage of apoptotic cells included both early- and late-stage apoptosis (Annexin V + PI − and Annexin V + PI+). Bar chart shows the percentage of apoptotic cells. N = 3 per group. Data are indicated as mean ± SD. For Fig. 5A, statistical analyses were performed using Student’s t test for paired comparisons between groups, For Fig. 5B-C, data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. Statistically significant at ***: p < 0.001 and ns denotes not significant. ARPE-19, spontaneously arising retinal pigment epithelium; H2O2, hydrogen peroxide; sEVs, small extracellular vesicles.
Functionality of interindividual BM-MSC-sEVs
To evaluate cytotoxicity of BM-MSCs-sEVs from each subject, ARPE-19 cells were incubated with 50 µg/mL of BM-MSC-sEVs for 24 h. The results showed that there were no significant differences among the BM-MSC-sEVs from each subject (Fig. 6A). In order to investigate whether variability BM-MSC-sEVs from different donors varied in their protective and curative effects on ARPE-19 cells against H2O2-induced cell damage, ARPE-19 cells were pretreated with BM-MSC-sEVs or challenged with H2O2 for 24 h prior to exposure to H2O2 or treatment with BM-MSC-sEVs (for another 24 h) (Fig. 6B-C and Supplementary Table 3). For sEVs-H2O2 group, the results found that the treatment with BM-MSC-sEVs from donors 1, 2, 4, and 5 significantly increased cell viability of ARPE-19 cells compared to H2O2-treated cells (33.11 ± 4.03%). Moreover, BM-MSC-sEVs from donor 1 was associated with the highest cell viability (58.00 ± 1.61%), followed by BM-MSC-sEV from donor 4 (56.90 ± 1.25%), donor 2 (52.50 ± 6.46%), donor 5 (46.50 ± 4.69%), and donor 3 (42.00 ± 4.69%), respectively (Fig. 6B). For H2O2-sEVs group, cell viabilities of ARPE-19 cells after the treatment of BM-MSC-sEVs from individual subject was significantly higher than those of H2O2-treated cells (33.11 ± 4.03%). BM-MSC-sEVs from donor 1 shown the highest cell viability (50.40 ± 1.30%), followed by BM-MSC-sEV from donor 5 (46.67 ± 2.71%), donor 4 (45.50 ± 4.25%), donor 2 (45.00 ± 2.21%), and donor 3 (42.57 ± 0.60%), respectively (Fig. 6C). Although the interindividual variability was found in the function of BM-MSC-sEVs, treatment with BM-MSC-sEVs from all donors could interrupt or revert the H2O2 damage process in ARPE-19 cells.
Fig. 6.
Variability comparison in the variables of BM-MSC-sEVs. (A) BM-MSC-sEVs from each subject were isolated from pooled CM at passages 3 through 6. ARPE-19 cells were co-incubated for 24 h with 50 µg/mL of BM-MSC-sEVs. Cell viability was measured by MTS assay. (B-C) ARPE-19 cells were challenged with 350 µM H2O2 for 24 h to induce cell damage. The effects of BM-MSC-sEVs were evaluated in ARPE-19 cells pre-treated with BM-MSC-sEVs for 24 h before H2O2 exposure (sEVs-H2O2) for another 24 h (B), and cells exposed to H2O2 for 24 h followed by BM-MSC-sEVs treatment for another 24 h (H2O2-sEVs) (C). Cell viability was measured by MTS assay. Data were analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. Statistically significant at *: p < 0.05, **: p < 0.01, ***: p < 0.001 and no asterisk denotes not significant. ARPE-19, spontaneously arising retinal pigment epithelium; H2O2, hydrogen peroxide; sEVs, small extracellular vesicles.
Discussion
BM-MSCs are among the most prevalent MSCs used in clinical therapy5,6. They play roles in the regeneration and immune systems, and in the treatment of various diseases including cardiovascular disease36, diabetes37, skin and eye diseases38,39 via the paracrine effects secreted by BM-MSCs, especially sEVs. The production and purification to GMP-grade of sEVs are essential steps in the development of sEVs as therapeutic vehicles. It is important to optimize the cell culture processes and sEV isolation methods in order to increase the production yield and purity of sEVs40. In this study, we investigated the upstream processes, beginning with BM-MSC culture through downstream processes including isolation, characterization, and efficiency testing of BM-MSC-sEVs.
The types of media used for cell culture are important not only in maintaining cell growth, but also in determining the properties of sEV secretion from the cells. There are many types of basal media for MSCs culture. Different basal media have different effects on cell growth, proliferation and differentiation potential23,41,42. It was found that both DMEM and α-MEM serve as appropriate basal medium for MSC based on morphology, CPDT analysis, and proliferative potency. BM-MSCs can be extensively cultured without losing their stem cell morphology at early passage. Our findings were in consistent with the previous work43.
Regarding the sEV isolation, there are several methods such as differential ultracentrifugation, filtration, precipitation, immunoaffinity capture, and chromatography44. These methods have limitations including low isolation yield, low purity, and time-consuming. Recently, TFF has been used for sEV isolation from large volume of cell culture medium45. Our TFF experiments for BM-MSC-sEV isolation were consistent with previous studies46,47, showing greater concentration and purity than with UC.
Recently, MSC-EVs have become a promising aspect of stem cell regenerative medicine. MSC-sEVs can transfer their cargo to recipient cells and mediate cellular activities under various physiological and pathological conditions9,10,47,48. A prior study suggested that MSC-EVs reduced reactive oxygen species generation, DNA damage, abnormal calcium signaling, and mitochondrial changes in keratinocytes of mouse skin exposed to H2O2 or UV radiation49. Another study revealed that human umbilical cord mesenchymal stem cell-derived extracellular vesicles could repair cisplatin-induced acute kidney injury in rats and rat renal tubular epithelial cells by promoting cell growth, and decreasing oxidative stress and cell death50. For retinal diseases, MSC-EVs protected retinal pigment epithelial cells from oxidative damage by regulating the Nrf2/Kepa1 signaling pathway, and maintaining the stability of retinal structures against sodium iodate-induced damage in rats51. Furthermore, MSC-EVs protected retinal pigment epithelial cells from light damage by decreasing VEGF-A expression in a rat model52. For retinitis pigmentosa, oxidative damage contributes to the death of rods, cones and retinal pigment epithelial cells3. Here, we induced ARPE-19 cells damage using H2O2 to examine whether BM-MSC-sEVs could protect ARPE-19 cells from oxidative injury. Our results indicated that they potentially had both protective and curative effects on H2O2 damage to ARPE-19 cells. Our preliminary results found that BM-MSC-sEV contain IL-6 and VEGF (data not shown), which are key regulators of antioxidant defenses. In addition, our proteomic analysis of BM-MSC-sEVs found ciliary neurotrophic factor (CNTF), which is a neuropoietic cytokine. CNTF binds to the receptors on photoreceptor cells and RPE cells, activating JAK/STAT pathway. The signaling cascade involved in cell growth, survival, and differentiation53,54. These protein contents in BM-MSC-sEVs may be integral to the mechanisms which protect ARPE-19 cells from oxidative damage. Further details of BM-MSC-sEV mechanisms in retinal diseases needed to be investigated.
Interindividual differences in the contents of sEVs may impact the efficacy of sEV-based therapies55. We found that sEVs isolated from different individuals vary in yield, and protective and curative effects on ARPE-19 cells. This is consistent with a previous study that evaluated the interindividual differences of human-induced pluripotent stem cell-derived cardiomyocyte impact on characteristic of sEV content and function. Those results showed that individuals differ in sEV miRNA expression and effect on endothelial cell function55. Further study will be needed to investigate individual genetics, sEV RNA profiles, and proteomics of sEVs to further understand the potential role of sEVs in retina treatment.
This study suffers from some potential limitations. First, we had a problem related to the primary cells derived from patients, such as RPE and photoreceptors. Differentiation batches of RPE and photoreceptors exhibited differences in tolerance to H2O2, resulting in inconsistent IC50 values across batches. Second, the development of patient-derived retinal organoids requires longer differentiation times and specialized handling. Due to this limitation, we used of ARPE-19 cells as an in vitro model for toxicity and functional testing. This may have influenced the outcomes, since the cultured cells did not closely mimic the in situ retinal environment. Third, we did not observe the safety and efficacy of sEVs in vivo. Many novel substances pass preclinical testing to enter human clinical trials, but then fail; approximately one-half of those failures are due to unanticipated human toxicity56. We are hopeful that in the near future we can develop a more realistic and representative model, like retinal organoid or organ-on-a-chip, for improving the accuracy and sensitivity of safety and efficacy assessments.
Materials and methods
Ethical approval
The protocol entitled “Production and characterization of GMP-BM-MSC-sEVs” was approved by the institutional review board of the Faculty of Medicine Siriraj Hospital, Mahidol University (COA number Si571/2022, dated 8 August 2022). The study was conducted in Siriraj Hospital, Mahidol University, Thailand. Involvement of human subjects adhered to the Declaration of Helsinki; each subject gave written informed consent.
Isolation, cultivation, and characterization of bone marrow-derived mesenchymal stem cells
Bone marrow (approximately 20 mL from the posterior iliac crest) of five adult subjects was aspirated into sterile heparin-containing syringes at Siriraj Hospital and then sent to the Department of Medical Sciences, Ministry of Public Health, Thailand. BM-MSCs were processed under controlled conditions in a cleanroom ISO 5 (class 100, Grade A). BM-MSCs were maintained in Dulbecco’s modified Eagles medium (DMEM) (Invitrogen, USA) supplemented with 10% hPL and antibiotic/antimycotic solution (Invitrogen, USA). Cells were incubated at 37 °C with 5% CO2. Culture medium was changed every 2 to 3 days. BM-MSCs were characterized by the presence of surface markers CD73, CD90, and CD105 and the lack of CD34, CD45, and human leukocyte antigen (HLA)-DR, as detected by flow cytometry (CytoFLEX S, Beckman Coulter, USA). BM-MSCs were also confirmed for their trilineage differentiation ability to adipocytes (StemPro™Adipogenesis Differentiation Kit), osteocytes (StemPro™Osteogenesis Differentiation Kit), and chondrocytes (StemPro™ Chondrogenesis Differentiation Kit, all three from Gibco, USA).
Investigation of phenotypic morphology and propagation of BM-MSCs
The third passage of BM-MSCs (3,000 cells/cm2) was cultured in DMEM and Minimum essential medium-alpha modification (α-MEM) (Invitrogen, USA) both supplemented with 10% sEVs-depleted human platelet lysate, antibiotic/antimycotic solution (Invitrogen, USA), and glutamine (Sigma, USA). Cells were incubated at 37 °C with 5% CO2. BM-MSCs were detached through treatment with 0.25% trypsin-EDTA (Gibco, USA) at 37 °C for 5 min, collected, and passaged at the aforementioned initial cell seeding density. Inverted microscopy was used during each expansion process to record cell morphology. To evaluate the proliferative capability of BM-MSCs, collected cells were counted using a trypan blue exclusion method at the end of each passage. CPDT was calculated using the following equation25.
 |
where  and
and  are the cell numbers at a specific time point (t) and at initial (i) seeding (day 0), respectively.
are the cell numbers at a specific time point (t) and at initial (i) seeding (day 0), respectively.
Isolation of small extracellular vesicles from bone marrow-derived mesenchymal stem cells
BM-MSCs were cultured until 50% confluence. The CM was then collected every two days until the cells reached 90% confluence. BM-MSC-sEVs were isolated from CM by differential UC (Beckman Coulter, USA) or TFF (Cytiva, USA). To prepare the CM for BM-MSC-sEVs isolation, CM was centrifuged at 500×g for 5 min and the supernatant was collected. After removing large-cell debris, CM was centrifuged at 1,500×g for 15 min, and at 20,000×g for 30 min to remove cellular debris, and microvesicles, respectively. Then CM was filtered through a 0.22 μm porous membrane (Corning, Germany) and collected.
For the UC method, 500 mL of filtrated CM was transferred to the ultracentrifuge tube and centrifuged at 110,000×g for 90 min using an L-80XP ultracentrifuge equipped with a Ti70 rotor (Beckman Coulter, USA). The pellet was suspended in 100 µL of PBS (Asalagen, Thailand) and ultracentrifuged again as described above. Then BM-MSC-sEV pellets were resuspended in 500 µL of PBS, filtered through a 0.22 μm porous membrane and stored as aliquots (100 µl).
For the TFF method, 500 mL of filtrated CM was subjected to a TFF system using 300 and 500 kDa membrane cut-off cartridges (Cytiva, USA). Speed of pump was adjusted to maintain a feed pressure (Pfeed) of 6 psi and a retentate pressure (Pretentate) of 4 psi. CM in the reservoir was concentrated at a rate of 2.0 to 2.5 mL/minute to the final volume of 10 mL. Concentrated sample was further diafiltrated using 20x volume of PBS. PBS containing concentrated sample was recirculated through the system for 10 min to detach sEVs that may have been lodged in the filter membrane. Then, the sample was concentrated to a final volume of 10 mL. PBS solution containing sEVs was filtered through a 0.22 μm porous membrane and stored as aliquots (100 µl).
For sEV storage, sEV samples were stored at −80 °C. For investigation of BM-MSC-sEVs characteristics, experiments were performed with in a week after isolation of BM-MSC-sEVs. For functional testing, all experiments used BM-MSC-sEVs which were stored at −80 °C for 2 weeks. Re-pelleted samples were not used in all experiments.
Characterization of small extracellular vesicles from bone marrow-derived mesenchymal stem cells
Concentration and size distribution of isolated particles were determined by NTA (NanoSight NS300; Malvern, UK). Samples were diluted in filtered PBS and injected into the instrument. Five 1-minute videos were acquired at ambient temperature.
The morphologies of isolated particles were visualized by TEM using a JEOL 1100 transmission electron microscope (JEOL, USA) at 60 kV. The isolated particles were fixed with 2% glutaraldehyde in PBS for 30 min at room temperature and loaded on a carbon-coated copper grid. After an incubation for 15 min, excess liquid was removed by filter paper. The isolated particles loaded on the grid were stained using 2% uranyl acetate and observed under TEM.
Total protein concentration of isolated particles was measured by bicinchoninic acid (BCA) assay (Pierce, Thermo Scientific, USA) according to the manufacture’s protocol. Common markers of sEVs were analyzed by western blotting. Protein from isolated particles (10 µg) was loaded onto 4% Bis-acrylamide gels (Thermo Fisher Scientific, USA). After transferring to polyvinylidene fluoride (PVDF) membranes (Bio-Rad, USA), they were washed and blocked with skim milk for 1 h. Then, the membranes were incubated overnight with primary antibodies (CD9: 25 kDa, Biolegend cat.312102; CD63: 65 kDa, Abcam ab215821; TSG101: 52 kDa, Abcam ab215821; and calnexin: 75 kDa, Biolegend cat.699402) at 4 °C. The next day, the membranes were washed and incubated with secondary antibody (For CD9 using goat anti-mouse, Biolegend cat. 405306; For CD63 and TSG101 using goat anti-rabbit, Abcam; For calnexin using goat anti-rat, Biolegend cat. 405405) for 1 h at room temperature. Images were captured using a biomolecular imager (ImageQuant LAS 4010, USA) according to the manufacturer’s instructions.
Sterility test
Sterility testing for aerobic and anaerobic bacteria, and fungi was performed using automate hemoculture with Becton Dickinson (BD) BECTEC™ PLUS + Anaerobic/F medium, BD BECTEC™ PED PLUS™/F medium, BD BECTEC™ Myco/F Lytic medium (BD, USA). Polymerase chain reaction was used for detection of mycoplasma contamination. Endotoxin was measured using a limulus amebocyte lysate kinetic method (PYROGENT™−5000 Kinetic Turbidimetric LAL Assay, Lonza, USA).
Cultivation of spontaneously arising retinal pigment epithelial cells
ARPE-19 cells were kindly provided by Assoc. Prof. Dr. Naravat Poungvarin (Mahidol University, Thailand). Those cells were obtained from the American Type Culture Collection (ATCC) (CRL-2302, Lot Number 70013110, passage 20; Manassas, VA). The twenty-fourth passage (P24) and P25 were used in this study. ARPE-19 cells were confirmed by short tandem repeat profiling at the Human Genetics Laboratory, Department of Pathology, Faculty of Medicine, Ramathibodi Hospital. Characteristics of the ARPE-19 cells were confirmed using a powerplex fusion6C system (Promega, USA). The results were identical to ARPE-19, CRL-2302 ATCC cell line (Supplementary Fig. 7). ARPE-19 cells were maintained in DMEM/F12 (Gibco, USA) supplemented with 10% heat-inactivated fetal bovine serum (Gibco, USA), antibiotic/antimycotic solution (Invitrogen, USA), and cultured in a humidified incubator at 37 °C with 5% CO2. Media exchange was performed twice a week.
Internalization of BM-MSC-sEVs into ARPE-19 cells
Our protocol was adapted from that described in a previous study57. BM-MSC-sEVs were labeled with PKH26 (PKH26 Red Fluorescent Cell Linker Kit, Sigma-Aldrich, USA). Prior to staining, 1 µM of PKH26 solution was added to 300 µL of diluent C and incubated at 37 °C for 15 min. Then, the pooled BM-MSC-sEV solution was added to PKH26 in diluent C. For control samples, PBS was used as the input instead of the BM-MSC-sEVs. BM-MSC-sEVs and control samples were stained according to the following procedures. To remove unbound dyes, the samples were ultracentrifuged at 110,000xg for 90 min at 4°C. Labeled BM-MSC-sEVs were gently suspended in complete medium. Prior to co-culture assay, ARPE-19 cells were seeded at a density of 1.5 × 104 cells/well into 24-well plates containing cover slips and incubated at 37 °C, overnight. The next day, the attached cells on cover slips were washed with PBS. Labeled BM-MSC-sEVs (50 µg/mL) were added to each well and incubated for 16 h. Then, the cells were washed with PBS. Cells were stained with 1xgreen fluorescent phalloidin-conjugated F-actin (ThermoFisher, USA) for 1 h at room temperature. After washing, cells were mounted using fluorescent-G with DAPI (Invitrogen, USA). Finally, the stained samples were imaged with a confocal laser scanning microscope (Nikon, Japan).
Live cell imaging
ARPE-19 cells were seeded at a density of 6 × 103 cells onto 8-well plates (ibidi, Germany) and incubated overnight at 37 °C. Two hours before imaging, cells were incubated with 50 µg/mL of PKH26-labeled pooled BM-MSC-sEVs diluted in complete medium. Cells were incubated with membrane-permeable dyes Hoechst 33,258 (final concentration of 10 µg/mL, ThermoFisher, USA) for 30 min at 37 °C before starting image acquisition. Cells were cultured at 37 °C and 5% CO2 in complete medium for 16 h. BM-MSC-sEV uptake into ARPE-19 cells was video captured by confocal microscopy (MICA, Leica, Germany) using a 20X objective lens. The resulting image stack was exported as AVI with a frame rate of 33 frames/sec. BM-MSC-sEVs were represented in red, while the nucleus was shown by Hoechst 33,258 in blue.
Co-culture assay
Co-culture of ARPE-19 cells and BM-MSC-sEVs were used for the studies of toxicity, curative, and protective effects. For toxicity testing, ARPE-19 cells were plated at a density of 3 × 103 cells/well in 96-well plates and incubated overnight at 37 °C. Cells were then mixed with different concentrations of BM-MSC-sEVs (25, 50, or 100 µg/mL) and incubated for 24 h.
For curative effect, ARPE-19 cells were seeded at a density of 3 × 103 cells/well in 96-well plates and incubated overnight at 37 °C. Then, cells were exposed to 350 µM H2O2 for 24 h. Culture medium was removed and cells were washed with PBS, followed by addition of culture medium containing BM-MSC-sEVs then incubated for 24 h.
For protective effect, ARPE-19 cells were plated at a density of 3 × 103 cells/well in 96-well plates and incubated overnight at 37 °C. The medium was then removed and replaced with culture medium containing BM-MSC-sEVs. Following another 24 h of incubation, culture medium was removed. ARPE-19 cells were then exposed to 350 µM H2O2 for 24 h. ARPE-19 cells in the culture medium alone and ARPE-19 cells exposed to H2O2 were served as controls. At the end of the experiments, cells were collected and processed for cell viability assessment.
Cell viability assay
Cell viability was determined using a 3-(4,5-dimethylthiazol-2-yl)−5-(3-carboxymethoxyphenyl)−2-(4-sulfophenyl)−2 H-tetrazolium (MTS) assay (Promega, USA). In brief, treatment media were replaced with new culture medium. Then, 20 µL of MTS solution was added into each well and incubated for 2 h at 37 °C. Absorbance was measured at 490 nm by a microplate reader (Omega Bio-Tek, USA). Cell viability was defined as the absorbance of treated wells divided by that of the control cells.
Cell proliferation assay
ARPE-19 cells were stained with 5-(and 6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) according to the manufacture’s protocol (CFSE Cell Division Tracker Kit, Biolegend, USA). Cells were plated in 24-well plates at a density of 1.5 × 105 cells with a volume of 500 µL/well. After 24 h, cells were treated with BM-MSC-sEVs isolated by either UC or TFF using 300- and 500-kDa membrane cut-offs at final concentrations of 0 and 50 µg/mL, respectively. After incubation for 5 days, cells were harvested and measured using a flow cytometer (FACSymphony A1, Becton Dickinson, USA). Data were analyzed using FlowJo software (Tree Star, USA). For each sample, 10,000 events were acquired. Unstained cells were used as controls to differentiate between CFSE signal and background noise.
Apoptosis detection test
ARPE-19 cells (2 × 105 cells/well in 24-well plates) were plated and incubated overnight at 37 °C. Cells were then exposed to 350 µM of H2O2 or treated with 50 µg/mL of sEVs. After 24 h, cells were treated with 50 µg/mL of sEVs or exposed to 350 µM of H2O2 for 24 h. Cells were harvested on day 3. To detect apoptosis, cells were washed twice and resuspended in 100 µL staining buffer, then stained with 5 µl of annexin V and 5 µL of 7AADs for 30 min at 37 °C in the dark (BioLegend, USA). After the incubation, 400 µL of staining buffer was added into each tube. Cells were acquired by a flow cytometer (FACSymphony A1). Data were analyzed by FlowJo software (Tree Star, USA). For each sample, 10,000 events were acquired. Cells were designated as follows: viable cells (Annexin V − PI−), early apoptotic cells (Annexin V + PI−), late apoptotic cells (Annexin V + PI+) and necrotic cells (Annexin V − PI+). Percentage of apoptotic cells included both early- and late-stage apoptosis (Annexin V + PI − and Annexin V + PI+).
Statistical analysis
Data are presented as mean ± standard deviation (SD). Statistical analyses of effect of culture medium on cultivation of BM-MSCs and BM-MSC-sEV secretion was performed using Student’s t-test for paired comparisons between groups. Comparison of BM-MSC-sEVs isolation by ultracentrifugation and tangential flow filtration and analysis of the effects of BM-MSC-sEVs on ARPE-19 cell viability and apoptosis were performed using one-way ANOVA followed by Tukey’s multiple comparison test using GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA). Statistical significance was defined as a p value of less than 0.05.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would also like to acknowledge and thank Dr. Panjaree Siwapornanan and Miss Narinee Srimark for their kind assistance in flow cytometer and NTA works. We also thanks Dr. Anyapat Atipimonpat, M.D. for her technical support on confocal microscopy.
Author contributions
LA, SS, AD, PB, PN: Bone marrow collection, BM-MSC isolation, characterization, expansion, quality control KP, NS, PS, AS: Optimization of BM-MSC-sEV production, isolation, and EV characterization KP, NS, PS, JP: Functions of BM-MSC-sEVs on ARPE-19 cellsLA, SS, KP, JP, NS: study conceptualization, experimental design, resources, data analysis LA, AT, SS, KP: SupervisionNS, JB, KP, AT, LA: Drafting or revising the manuscriptAll authors have read and approved the final version of the manuscript.
Funding
This work was supported by Siriraj Hospital, Mahidol University, the Siriraj Foundation (D1671), and the National Research Council of Thailand (NRCT): High-Potential Research Team Grant Program, grant number N42A650870.
Data availability
The data used to support the findings of this study are included within the article and supplementary file.
Declarations
Competing interests
The authors have no commercial, proprietary or financial interest in the products or companies described in this article. The authors have not use AI-generated work in this manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
La-ongsri Atchaneeyasakul, Email: laongsri.ath@mahidol.ac.th, Email: atchanee@hotmail.com.
Kovit Pattanapanyasat, Email: kovit.pat@mahidol.ac.th.
References
- 1.Duncan, J. L. et al. Inherited retinal degenerations: current landscape and knowledge gaps. Transl Vis. Sci. Technol.710.1167/tvst.7.4.6 (2018). [DOI] [PMC free article] [PubMed]
- 2.Nguyen, X. T. et al. Retinitis pigmentosa: current clinical management and emerging therapies. Int. J. Mol. Sci.24, 7481. 10.3390/ijms24087481 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang, J. et al. Role of oxidative stress in retinal disease and the early intervention strategies: A review. Oxid. Med. Cell. Longev.2022 (7836828-13). 10.1155/2022/7836828 (2022). [DOI] [PMC free article] [PubMed]
- 4.Adak, S., Magdalene, D., Deshmukh, S., Das, D. & Jaganathan, B. G. A review on mesenchymal stem cells for treatment of retinal diseases. Stem Cell. Reviews Rep.17, 1154–1173. 10.1007/s12015-020-10090-x (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Margiana, R. et al. Clinical application of mesenchymal stem cell in regenerative medicine: a narrative review. Stem Cell Res. Ther.13, 366. 10.1186/s13287-022-03054-0 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holan, V., Palacka, K. & Hermankova, B. Mesenchymal stem Cell-Based therapy for retinal degenerative diseases: experimental models and clinical trials. Cells10, 588. 10.3390/cells10030588 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holan, V., Hermankova, B., Krulova, M. & Zajicova, A. Cytokine interplay among the diseased retina, inflammatory cells and mesenchymal stem cells - a clue to stem cell-based therapy. World J. Stem Cells. 11, 957–967. 10.4252/wjsc.v11.i11.957 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuekprakhon, A. et al. Intravitreal autologous mesenchymal stem cell transplantation: a non-randomized phase I clinical trial in patients with retinitis pigmentosa. Stem Cell. Res. Ther.12, 52. 10.1186/s13287-020-02122-7 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma, V. & Mukhopadhyay, C. D. Exosome as drug delivery system: current advancements. Extracell. Vesicle. 3, 100032. 10.1016/j.vesic.2023.100032 (2024). [Google Scholar]
- 10.Baruah, H., Sarma, A., Basak, D., Das, M. & Exosome From biology to drug delivery. Drug Deliv Transl Res.1410.1007/s13346-024-01515-y (2024). 1480 – 516. [DOI] [PubMed]
- 11.Tan, F. et al. Clinical applications of stem cell-derived exosomes. Signal. Transduct. Target. Therapy. 9, 17. 10.1038/s41392-023-01704-0 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hade, M. D., Suire, C. N. & Suo, Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine. Cells. 10, ; (1959). 10.3390/cells10081959 (2021). [DOI] [PMC free article] [PubMed]
- 13.Park, J. H. Regulation of in vivo fate of exosomes for therapeutic applications: new frontier in nanomedicines. J. Control Release. 348, 483–488. 10.1016/j.jconrel.2022.05.058 (2022). [DOI] [PubMed] [Google Scholar]
- 14.Roszkowski, S. Therapeutic potential of mesenchymal stem cell-derived exosomes for regenerative medicine applications. Clin. Exp. Med.24, 46. 10.1007/s10238-023-01282-z (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mead, B., Ahmed, Z. & Tomarev, S. Mesenchymal stem Cell-Derived small extracellular vesicles promote neuroprotection in a genetic DBA/2J mouse model of glaucoma. Invest. Ophthalmol. Vis. Sci.59, 5473–5480. 10.1167/iovs.18-25310 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Safwat, A. et al. Adipose mesenchymal stem cells-derived exosomes attenuate retina degeneration of streptozotocin-induced diabetes in rabbits. J. Circ. Biomark.7, 1849454418807827. 10.33393/jcb.2018.2096 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathew, B. et al. Mesenchymal stem cell-derived extracellular vesicles and retinal ischemia-reperfusion. Biomaterials19710.1016/j.biomaterials.2019.01.016 (2019). 146 – 60. [DOI] [PMC free article] [PubMed]
- 18.Moisseiev, E., Anderson, J. D., Oltjen, S., Goswami, M., Zawadzki, R. J., Nolta,J. A., et al. Protective Effect of Intravitreal Administration of Exosomes Derived from Mesenchymal Stem Cells on Retinal Ischemia. Curr Eye Res. 42, 1358–1367;; https://doi.org/10.1080/02713683.2017.1319491 (2017). [DOI] [PMC free article] [PubMed]
- 19.Bai, L. et al. Effects of mesenchymal stem Cell-Derived exosomes on experimental autoimmune uveitis. Sci. Rep.7, 4323. 10.1038/s41598-017-04559-y (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, J. Y. et al. Comparative analysis of MSC-Derived exosomes depending on cell culture media for regenerative bioactivity. Tissue Eng. Regen Med.18, 355–367. 10.1007/s13770-021-00352-1 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holcar, M. et al. Comprehensive phenotyping of extracellular vesicles in plasma of healthy Humans - Insights into cellular origin and biological variation. J. Extracell. Vesicles. 14, e70039. 10.1002/jev2.70039 (2025). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ben Azouna, N. et al. Phenotypical and functional characteristics of mesenchymal stem cells from bone marrow: comparison of culture using different media supplemented with human platelet lysate or fetal bovine serum. Stem Cell. Res. Ther.3, 6. 10.1186/scrt97 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhanasekaran, M. et al. A comprehensive study on optimization of proliferation and differentiation potency of bone marrow derived mesenchymal stem cells under prolonged culture condition. Cytotechnology65, 187–197. 10.1007/s10616-012-9471-0 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kusuma, G. D. et al. Effect of 2D and 3D culture microenvironments on mesenchymal stem Cell-Derived extracellular vesicles potencies. Front. Cell. Dev. Biol.10, 819726. 10.3389/fcell.2022.819726 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang, Y. H. K., Ogando, C. R., Wang See, C., Chang, T. Y. & Barabino, G. A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther.9, 131. 10.1186/s13287-018-0876-3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao, M., Cai, J., Zitkovsky, H. S., Chen, B. & Guo, L. Comparison of Yield, Purity, and functional properties of Large-Volume exosome isolation using ultrafiltration and Polymer-Based precipitation. Plast. Reconstr. Surg.149, 638–649. 10.1097/PRS.0000000000008830 (2022). [DOI] [PubMed] [Google Scholar]
- 27.Kim, J. Y. et al. Defined MSC exosome with high yield and purity to improve regenerative activity. J. Tissue Eng.12, 20417314211008626. 10.1177/204173142110086 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lobb, R. J. et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles. 4, 27031. 10.3402/jev.v4.27031 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng, L., Zhang, K., Wu, S., Cui, M. & Xu, T. Focus on mesenchymal stem Cell-Derived exosomes: opportunities and challenges in Cell-Free therapy. Stem Cells Int.2017 (6305295). 10.1155/2017/6305295 (2017). [DOI] [PMC free article] [PubMed]
- 30.Cai, H., Gong, J., Abriola, L., Hoyer, D. & Noggle, T. Nyscf Global Stem Cell Array, S., et al. High-throughput screening identifies compounds that protect RPE cells from physiological stressors present in AMD. Exp Eye Res. 185, 107641; (2019). 10.1016/j.exer.2019.04.009 [DOI] [PubMed]
- 31.Samuel, W. et al. Appropriately differentiated ARPE-19 cells regain phenotype and gene expression profiles similar to those of native RPE cells. Mol. Vis.23, 60–89 (2017). [PMC free article] [PubMed] [Google Scholar]
- 32.Welsh, J. A. et al. Minimal information for studies of extracellular vesicles (MISEV2023): from basic to advanced approaches. J. Extracell. Vesicles. 13, e12404. 10.1002/jev2.12404 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akbar, A., Malekian, F., Baghban, N., Kodam, S. P. & Ullah, M. Methodologies to isolate and purify clinical grade extracellular vesicles for medical applications. Cells11, e12404. 10.3390/cells11020186 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warnecke, A. et al. First-in-human intracochlear application of human stromal cell-derived extracellular vesicles. J. Extracell. Vesicles. 10, e12094. 10.1002/jev2.12094 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zarrabi, M. et al. Allogenic mesenchymal stromal cells and their extracellular vesicles in COVID-19 induced ARDS: a randomized controlled trial. Stem Cell Res. Ther.14, 169. 10.1186/s13287-023-03402-8 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Rhijn-Brouwer, F. C. C., Gremmels, H., Fledderus, J. O. & Verhaar, M. C. Mesenchymal stromal cell characteristics and regenerative potential in cardiovascular disease: implications for cellular therapy. Cell. Transpl.27, 765–785. 10.1177/0963689717738257 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azizi, Z. et al. Bone marrow mesenchymal stromal cells for diabetes therapy: touch, fuse, and fix? Stem Cell Res. Ther.13, 348. 10.1186/s13287-022-03028-2 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ding, S. L. S., Kumar, S. & Mok, P. L. Cellular reparative mechanisms of mesenchymal stem cells for retinal diseases. Int. J. Mol. Sci.18, 1406. 10.3390/ijms18081406 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mannino, G. et al. Potential therapeutic applications of mesenchymal stem cells for the treatment of eye diseases. World J. Stem Cells. 13, 632–644. 10.4252/wjsc.v13.i6.632 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takakura, Y. et al. Quality and safety considerations for therapeutic products based on extracellular vesicles. Pharm. Res.41, 1573–1594. 10.1007/s11095-024-03757-4 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikolits, I., Nebel, S., Egger, D., Kreß, S. & Kasper, C. Towards physiologic culture approaches to improve standard cultivation of mesenchymal stem cells. Cells10 (886). 10.3390/cells10040886 (2021). [DOI] [PMC free article] [PubMed]
- 42.Sotiropoulou, P. A., Perez, S. A., Salagianni, M., Baxevanis, C. N. & Papamichail, M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 24, 462–471. 10.1634/stemcells.2004-0331 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Czapla, J. et al. The effect of culture media on large-scale expansion and characteristic of adipose tissue-derived mesenchymal stromal cells. Stem Cell Res. Ther.10, 235. 10.1186/s13287-019-1331-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao, J. et al. Recent developments in isolating methods for exosomes. Front. Bioeng. Biotechnol.10, 1100892. 10.3389/fbioe.2022.1100892 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paganini, C. et al. Scalable production and isolation of extracellular vesicles: available sources and lessons from current industrial bioprocesses. Biotechnol. J.14, e1800528. 10.1002/biot.201800528 (2019). [DOI] [PubMed] [Google Scholar]
- 46.Busatto, S. et al. Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cells7, 273. 10.3390/cells7120273 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haraszti, R. A. et al. Exosomes produced from 3D cultures of MSCs by tangential flow filtration show higher yield and improved activity. Mol. Ther.26, 2838–2847. 10.1016/j.ymthe.2018.09.015 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kourembanas, S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annu. Rev. Physiol.77, 13–27. 10.1146/annurev-physiol-021014-071641 (2015). [DOI] [PubMed] [Google Scholar]
- 49.Wang, T. et al. MSC-derived exosomes protect against oxidative stress-induced skin injury via adaptive regulation of the NRF2 defense system. Biomaterials257, 120264 10.1016/j.biomaterials.2020.120264 (2020). [DOI] [PubMed]
- 50.Zhou, Y. et al. Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res. Ther.4, 34. 10.1186/scrt194 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang, Y., Kang, Y., Zhang, X. & Cheng, C. Mesenchymal stem cell exosomes as nanotherapeutics for dry age-related macular degeneration. J. Control Release. 357, 356–370. https://doi.org/0.1016/j.jconrel.2023.04.003 (2023). [DOI] [PubMed] [Google Scholar]
- 52.He, G. H. et al. Mesenchymal stem cells-derived exosomes ameliorate blue light stimulation in retinal pigment epithelium cells and retinal laser injury by VEGF-dependent mechanism. Int. J. Ophthalmol.11, 559–566. 10.18240/ijo.2018.04.04 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li, R., Wen, R., Banzon, T., Maminishkis, A. & Miller, S. S. CNTF mediates neurotrophic factor secretion and fluid absorption in human retinal pigment epithelium. PLoS One. 6, e23148. 10.1371/journal.pone.0023148 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nystuen, A., Gonzalez-Lopez, E., Kauper, K. A., Eade, K. & Aaberg, T. M. Jr. Neuroprotective properties of ciliary neurotrophic factor in the retina for the treatment of macular telangiectasia type 2. Cytokine Growth Factor. Rev.84, 12–19. 10.1016/j.cytogfr.2025.06.005 (2025). [DOI] [PubMed] [Google Scholar]
- 55.Turner, A. et al. Donor-specific phenotypic variation in HiPSC cardiomyocyte-derived exosomes impacts endothelial cell function. Am. J. Physiol. Heart Circ. Physiol.320, H954–H68. 10.1152/ajpheart.00463.2020 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van Norman, G. A. Limitations of animal studies for predicting toxicity in clinical trials: is it time to rethink our current approach? JACC Basic. Transl Sci.4, 845–854. 10.1016/j.jacbts.2019.10.008 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lertjuthaporn, S. et al. Extracellular vesicles from naegleria fowleri induce IL-8 response in THP-1 macrophage. Pathogens11, 632. 10.3390/pathogens11060632 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are included within the article and supplementary file.