Abstract
Introduction
Adolescent substance use (SU) rates remain high, speaking to continued need for enhanced insight into etiological factors. While working memory-related task performance and brain activity have been highlighted as potential predictors, mechanistic links to SU remain unclear. One possible link explored here is alexithymia, which is characterized by difficulty describing, identifying, and recognizing emotions and associated with altered prefrontal cortex (PFC) and superior temporal gyrus (STG) activity.
Methods
Adolescents (n = 137) from a longitudinal study completed a n-back working memory (WM) task during fMRI scanning at baseline. Utilizing serial mediation models, we considered the interrelations between WM-related brain activity (i.e., lateral PFC [lPFC], STG), task performance (i.e., d-prime), Toronto Alexithymia Scale scores (i.e., difficulty describing feelings), and self-reported SU variables at follow-up. Six models estimated the influence of lPFC/STG activity on e-cigarette, cannabis, and alcohol use via task performance and alexithymia.
Results
In the absence of serial mediation, we observed a simple mediation effect linking brain activity and SU via alexithymia. Specifically, less lPFC WM-related activation was linked with higher alexithymia which, in turn, predicted more e-cigarette use assessed over a year later. Conversely, less STG deactivation was linked with higher alexithymia, which predicted more e-cigarette use. Significant indirect effects were not detected in the cannabis or alcohol models.
Conclusions
These outcomes highlight alexithymia as a mechanistic link between WM abilities and e-cigarette use. Functional alterations in WM-related brain regions may render some adolescents prone to difficulty communicating feelings and potential nicotine use to modulate emotions or connect socially.
Keywords: E-cigarettes, Cannabis, Alcohol, FMRI, Adolescence, Working memory, Alexithymia
Highlights
-
•
The impact of working memory alterations on teen substance use was examined.
-
•
Alexithymia mediated the effect of working memory brain activity on e-cigarette use.
-
•
Teens with higher alexithymia may use e-cigarettes as a way to cope.
-
•
Working memory training may offer benefits for affect regulation.
1. Introduction
While adolescent substance use (SU) has declined since the COVID-19 pandemic (Miech, Johnston, Patrick, O'Malley, et al., 2024), rates remain high. Monitoring the Future Study estimates indicate that 21 % of adolescents engaged in past year e-cigarette, 18 % in cannabis, and 30 % in alcohol use (Miech, Johnston, Patrick, and O'Malley, 2024). These rates are concerning given negative health consequences and the potential for problematic use to develop among vulnerable individuals (Feeney and Kampman, 2016, Guerri and Pascual, 2010, Livingston et al., 2022, Sutherland et al., 2016). Therefore, delineating interrelations between etiological factors influencing adolescent SU at the neurobiological, cognitive, and affective levels remains a priority (Green et al., 2024). An enhanced perspective on such interrelations holds promise for improving intervention efforts.
Neurobiologically, adolescents may engage in risky behaviors (e.g., SU) given the differential developmental trajectories of two brain systems influencing decision-making (Casey et al., 2008, Steinberg, 2008). Subcortical limbic structures implicated in pursuing rewarding experiences are thought to develop more quickly than prefrontal regions implicated in cognitive control and self-regulation (Hammond et al., 2014, Shulman et al., 2016). As control-related prefrontal cortex (PFC) regions mature later, they may be particularly relevant for adolescent risk-taking behaviors (Hammond et al., 2014). Indeed, altered maturational trajectories in the PFC may impact addiction vulnerability (Hammond et al., 2014). Yet, less work has focused on mechanistic paths to adolescent SU linking brain, cognitive, and emotional factors.
From a cognitive perspective, working memory (WM) abilities predict adolescent SU (Grenard et al., 2008) such that lower WM task performance is linked with earlier use progression and disorder diagnosis among adolescents (Khurana et al., 2017). Conversely, youth with stronger WM tend toward less impulsivity (Khurana et al., 2017) and may possess greater self-control placing more value on long-term goals as opposed to short-term rewards (Daly et al., 2016, Duckworth and Steinberg, 2015). WM abilities are also linked with other mental operations which may influence decision-making such as impulse control, reasoning, and emotion regulation during adolescence (Grenard et al., 2008). Notably, most work examining a link between WM and adolescent SU has been based on WM behavioral tasks (Grenard et al., 2008, Khurana et al., 2017), and more empirical research is needed regarding the interrelation between WM-related brain activity and adolescent SU. Optimal WM performance is associated with increased activity in the lateral prefrontal (lPFC), dorsomedial prefrontal (dmPFC), and parietal cortices (i.e., central executive network [CEN] regions) and concomitant decreased activity across regions including the ventromedial (vm)PFC, posterior cingulate cortex (PCC), and superior temporal gyrus (STG) (i.e., default-mode network [DMN] regions). During cognitively demanding tasks, both suboptimal activation of CEN regions and suboptimal deactivation of DMN regions accompanies reduced WM task performance (Hu et al., 2013), increased attentional lapses (Hinds et al., 2013, Weissman et al., 2006), and link with SU (Loughead et al., 2010, Loughead et al., 2015). CEN and DMN regions are also linked with emotional processing in adults (Kohn et al., 2014, Li et al., 2014, Wilcox et al., 2016). For example, hypoactivity in PFC regions influencing affective processing (Nejati et al., 2021) and hyperactivity in DMN regions influencing rumination (Zhou et al., 2020) may contribute to negative affect, emotion dysregulation, and SU (Wilcox et al., 2016). Although less work has examined such findings among adolescents, alterations in PFC regulatory regions during childhood and adolescence predicts future SU (George and Koob, 2010). Notably, emotion dysregulation, which represents an impairment in attention, information-processing and reasoning, is considered one pathway to addiction (George and Koob, 2010). The normative maturational-delay of PFC regions, which are underdeveloped during adolescence compared to limbic structures implicated in reward (Casey et al., 2008, Steinberg, 2008), may also influence the cognitive processing and awareness of emotions, increasing SU risk. Plausibly, psychological constructs related to emotion regulation challenges could be mediators between WM-related brain activity and adolescent SU.
Alexithymia is a psychological construct characterized by difficulty describing feelings, identifying feelings, and recognizing others’ emotions (Kano and Fukudo, 2013, Kopera et al., 2015, Sifneos, 1973). Such difficulties potentially stem from the interplay of emotion-related experiential processes and the cognitive processing of those experiences (Luminet et al., 2021). Alexithymia is linked with functional alterations in brain regions involved in affective, regulatory, and cognitive operations (Dai et al., 2018, Riadh et al., 2019). For example, young adults high in alexithymia (i.e., top quartile on the Toronto Alexithymia Scale [TAS]), show lPFC hypoactivity during empathy-related tasks (e.g., when viewing others in pain; (Moriguchi et al., 2007, Taylor and Bagby, 2004). During emotional face processing, alterations in the STG, a region linked with social cognition, emotional understanding, and self-referential information (Bigler et al., 2007, Zevin, 2009), are also observed among adults characterized by high versus low alexithymia (Kano and Fukudo, 2013, Kano et al., 2003). Noteworthy, STG activity during emotional face processing negatively correlates with alexithymia scores (Reker et al., 2010). While alexithymia studies have characterized lPFC and STG alterations during affective processing among adults, less work has considered cognitive processing (Correro et al., 2021), especially among adolescents. Indeed, alexithymia’s potential cognitive manifestations are underappreciated (Riadh et al., 2019) despite emerging evidence highlighting reduced performance in WM and other executive function tasks (Frawley and Smith, 2001, Koven and Thomas, 2010, Riadh et al., 2019, Vermeulen et al., 2018). More recently, alexithymia has been conceptualized as an alteration of emotional representations in WM (Frawley and Smith, 2001). Consistent with this conceptualization, higher alexithymia characteristics are influenced by impaired WM (Frawley and Smith, 2001, Koven and Thomas, 2010), indirectly suggesting suboptimal cognitive processing of emotional experiences (Correro et al., 2021, Moriguchi et al., 2007). Higher alexithymia also correlates with reduced performance during executive function tasks assessing inhibition, flexibility, planning, and cognitive conflict resolution (Riadh et al., 2019, Vermeulen et al., 2018, Zhang et al., 2011). Neurobiologically, alexithymia has been characterized as a frontal lobe impairment, consistent with implications for executive functions such as WM (Koven and Thomas, 2010, Luminet et al., 2021). While direct developmental studies are limited, these previous observations support the premise that altered lPFC activity in adolescents may contribute to affective and/or cognitive manifestations linked with greater alexithymia. The integration between affective and cognitive processes such as cognitively reappraising emotional stimuli (Golkar et al., 2012), may be more limited at this developmental stage given the normative maturational-delay of PFC regions (Casey et al., 2008, Steinberg, 2008) thereby reinforcing alexithymia characteristics. In other words, adolescents with alexithymia may struggle to cognitively reappraise, describe, or even identify elicited emotions. Alexithymia in turn may increase vulnerability to maladaptive coping strategies, such as SU.
Alexithymia may also confer risk for youth SU (Dorard et al., 2017, Sutherland et al., 2022). Our prior work showed that difficulty describing feelings (but not identifying or recognizing others’ emotions) predicted adolescent e-cigarette use assessed over a year later (Sutherland, Fallah-Sohy, et al., 2022). Regarding cannabis, alexithymia scores are higher among youth diagnosed with disordered use versus non-users (Dorard et al., 2017). Regarding alcohol, difficulty identifying feelings has been linked with more use among younger (<13 years), but curiously, not older adolescents (14–18 years) (Gatta et al., 2014). Despite these observations, some data suggest that alexithymia assessed during adolescence may not predict later SU disorder in adulthood (Patwardhan et al., 2019). Nonetheless, adolescents higher in alexithymia may experience distress resulting from difficulty processing emotional states and engage in SU as a coping mechanism (Kopera et al., 2020). Given our prior observation of a link between difficulty describing feelings and youth SU (Sutherland, Fallah-Sohy, et al., 2022), we focused on this facet of alexithymia and interrogated its mediating role between WM-related brain and performance measures with subsequent e-cigarette, cannabis, and alcohol use.
We examined WM-related brain activity, WM-related task performance, and self-reported alexithymia as mechanistic paths contributing to adolescent SU. Given that optimal WM performance necessitates sufficient CEN activation and DMN deactivation, we focused on task-related brain regions within these networks which also have been linked with alexithymia (i.e., lPFC and STG; (Kano and Fukudo, 2013, Moriguchi et al., 2007, Nadeau, 2021, Reker et al., 2010). We considered three hypotheses pertaining to task-, alexithymia-, and SU-related outcomes. Regarding task-related outcomes, we hypothesized that as WM-load increased, WM-related task performance would decrease and that lPFC activations and STG deactivations would increase. Regarding alexithymia outcomes, we hypothesized that reduced lPFC activations and STG deactivations, as well as reduced WM-related task performance, would correlate with greater difficulty describing feelings. Regarding SU-related outcomes, we hypothesized that WM-related task performance and/or difficulty describing feelings would mediate the association between WM-related brain activity and e-cigarette, cannabis, and/or alcohol use across separate serial medial models. As prior alexithymia studies have generally considered brain activity (Bigler et al., 2007, Dai et al., 2018, Moriguchi et al., 2007, Taylor and Bagby, 2004, Zevin, 2009) or behavioral performance measures separately(Frawley and Smith, 2001; Koven and Thomas, 2010; Riadh et al., 2019; Vermeulen et al., 2018), we viewed the inclusion of both WM-related brain and performance variables in these serial mediation models as an extension of prior work.
2. Methods
2.1. Participants
We assessed data from a subsample of 137 adolescents (48 % female, 88 % White, 85 % Hispanic/Latino[a], Mage=14.9) who completed wave-1 (W1, baseline) and wave-2 (W2, follow-up ∼15 months later) of a larger longitudinal study (N = 264). While a subsample was assessed here, as not all participants completed an MRI scan, this subgroup did not differ from the full sample in terms of demographic or SU variables (p’s range: 0.15–0.99). At enrollment, participants were high school freshmen or sophomores and their caregivers. W1 data collection spanned March 2018 through December 2019 and W2 from June 2019 to June 2021. Eligibility criteria for youth included English-fluency and no diagnosis of a learning disorder, intellectual/physical disability, neurological condition, or severe mental illness. MRI exclusionary criteria included left-handedness, non-removable metal, and pregnancy among females.
2.2. Study procedures
Recruitment occurred at public schools and families interested in participating were contacted for screening. Eligible individuals were then scheduled for an initial visit (W1a). Following consent/assent, youths and caregivers completed questionnaires in separate rooms for ∼1.5hrs and ∼45 min, respectively. For those eligible, W1 data collection also involved a second visit (W1b) consisting of additional youth questionnaires (∼15 min) and an MRI scan (∼90 min), scheduled within one month of the W1a visit. Two tasks were completed during the MRI session (i.e., a WM and monetary incentive delay task) with a 10 min resting-state scan in-between. W2 assessments involved similar procedures as W1a, however given COVID-19 restrictions, some visits were completed remotely. Questionnaires were administered via REDCap (& consortium, R., 2019, Harris et al., 2009) using a tablet (in-person) or personal electronic devices (remote visits). Study procedures were approved by the Institutional Review Board and participants were compensated after each visit. Additional participant and procedure details are reported elsewhere (Cristello et al., 2020, Hartmann et al., 2021, Sutherland et al., 2022, Sutherland et al., 2022, Trucco et al., 2021).
2.3. Self-report measures
During visit W1b, adolescents completed the TAS (Bagby et al., 1994) to assess difficulty describing feelings (e.g., “I am able to describe my feelings easily”; Cronbach’s α=0.79). We focused on this facet of alexithymia given prior associations with executive functions (Vermeulen et al., 2018) and adolescent SU (Sutherland, Fallah-Sohy, et al., 2022). Items on the TAS were rated on a 5-point Likert scale and responses were summed yielding subscale scores with higher values reflecting greater difficulty.
E-cigarette, cannabis, and alcohol use were assessed at W2 using a continuous item for each substance adapted from the Population Assessment of Tobacco and Health (PATH) Survey (Hyland et al., 2016). Participants were asked “Since your last visit, on how many days did you [use an Electronic Nicotine Delivery System product / use cannabis / have one or more alcoholic drinks]?” As our main outcomes pertained to e-cigarette use (see results), we conducted an ancillary analysis utilizing data from the Adolescent E-cigarette Consequences Questionnaire (AECQ; Figure S2; (Cristello et al., 2020), which quantifies use outcome expectancies and allowed us to consider youth’s self-reported reasons for use. Outcome expectancies, which can be positive (e.g., negative affect reduction) or negative (e.g., health consequences), are associated with anticipated consequences that result from a certain behavior (Cristello et al., 2020, Heinz et al., 2010, Reesor et al., 2017). We used the AECQ’s negative affect reduction subscale given prior work indicating that youth commonly report vaping to cope with such feelings (Kong et al., 2019).
2.4. Working memory task
Participants completed a letter n-back task with varying WM-loads (Owen et al., 2005). The task consisted of three 5-minute runs each with four levels (i.e., 0-, 1-, 2-, 3-back) during which participants saw letters serially presented. During the 0-back level, participants were instructed to button press (right index finger) when a predefined target (i.e., “D” or “d”) was presented and to avoid pressing for other letters. For other levels, participants were instructed to button press if the current letter matched the letter presented one item ago (1-back), two items ago (2-back), or three items ago (3-back), which required the maintenance of increasingly more items in WM. Task runs consisted of 8 blocks, each starting with the 0-back level after which, 1-, 2-, and 3-back levels were pseudo-randomly delivered. A 2-sec visual instruction informed participants of the current condition (Hu et al., 2013). Performance was quantified by d-prime at each level and calculated as hit rate (proportion of trials when the target was present and participant responded) minus false alarm rate (when the target was not present and participant responded (Haatveit et al., 2010). Defined as extreme outliers, participants were excluded if d-prime values fell outside of the range of ‘Q3 + 3*IQR’ or ‘Q1 - 3*IQR’. Prior to completing this task in the MRI, participants were also carefully trained on the task and required to correctly respond on 60 % or more trials within each difficulty level. As a result, 6 participants were removed based on low performance. To assess performance, repeated measures ANOVA was conducted in R (R Studio Team, 2020) followed by Tukey-corrected post-hoc t-tests. To characterize interrelations between performance and alexithymia, linear regressions were conducted in R covarying for age, sex, and ethnicity.
2.5. fMRI data
Data were acquired on a 3 T Siemens MAGNETOM Prisma scanner with a 32-channel head coil. During the task, 60 slices (2.4 mm) were obtained using a multi-slice gradient-echo, echo-planar imaging sequence sensitive to BOLD effects (repetition time [TR]=800ms, echo time [TE]=30ms, flip angle [FA]=52°, voxel size=2.4mm3, field of view [FOV]=216 mm, multiband acceleration=6). Structural T1-weighted images were acquired using a MPRAGE sequence (TR=2500 ms; TE=2.9ms; FA=8°; voxel size=1mm3). Data were preprocessed with fMRIPrep 20.2.1 (Esteban et al., 2017, Esteban et al., 2018) and analyzed with AFNI (Cox and Hyde, 1997) according to recommended best practices (Esteban et al., 2019, Nichols et al., 2017). Preprocessing steps included: skull-stripping, distortion correction, co-registration, motion correction, slice-time correction, tissue segmentation, and spatial normalization (details in supplement).
AFNI’s 3dDeconvolve and 3dREMLfit (v20.2.10) were used to conduct subject-level, voxel-wise linear regressions using six motion-correction parameters as covariates and including task-related regressors for the 0-, 1-, 2-, and 3-back conditions. WM-related activity was estimated by contrasting the 1-, 2-, and 3-back levels against the 0-back via general linear contrasts. Subject-level β-weights from these contrasts were submitted to a group-level, whole-brain ANOVA (3dMVM) to identify voxel’s sensitive to the WM-load manipulation while controlling for age, sex, and mean framewise displacement (FD). The group-level WM-load map was thresholded at a cluster-corrected level of pFWE-corrected< 0.001 (pvoxel-wise<1e-6, cluster extent: 7 voxels; via 3dClustSim with spatial autocorrelation correction). Task-related ROIs were labeled using AFNI’s whereami function in consultation with the EBRAIN atlases (https://www.ebrains.eu/tools/siibra-explorer). Average β-weights for each task-related ROI were extracted (3dROIstats) for graphical examination and follow-up analyses. To characterize the nature of WM-load effects within ROIs, Tukey-corrected t-tests were conducted in R. To characterize interrelations between brain (de)activations and alexithymia, linear regressions were conducted in R covarying for age, sex, and ethnicity.
2.6. Serial mediation models
Descriptive statistics and correlations across variables were first calculated. Structural equation modeling was then used to estimate serial mediation using R’s lavaan package (Rosseel, 2012). Six separate models assessed whether WM-related task performance (M1: d-prime) and/or alexithymia (M2: difficulty describing feelings) mediated the impact of WM-related brain (de)activations (lPFC and STG; X) on e-cigarette, cannabis, or alcohol use (Y). Study variables were normally distributed (skewness range: −1.9–2.1; kurtosis: −2.0–1.9) except the SU variables (skewness: 5.1–7.3; kurtosis: 27.8–58.8). As such, 95 % bootstrap based on 1000 samples were utilized which considers nonnormality by adjusting parameter estimates of standard errors and confidence intervals (CIs; (Preacher and Hayes, 2008). Covariates included age, sex, ethnicity, and baseline lifetime use of the substance of interest. To determine if observed outcomes were accounted by variation of in-scanner head motion (Satterthwaite et al., 2019), we performed ancillary serial mediations including FD as a covariate. Lastly, to enhance interpretation of potential reasons for e-cigarette use, additional models explored links between brain (de)activations (X), task performance (M1), alexithymia (M2) and self-reported outcome expectancies of using e-cigarettes (Y: AECQ scores).
3. Results
3.1. WM-related task performance outcomes
Repeated measures ANOVA evaluated WM-load effects comparing WM-related task performance across n-back levels (Fig. 1, left). As expected, d-prime differed by level where performance decreased as difficulty increased (F[2322]=237.7, p < 0.001). To characterize relations between performance and alexithymia, we conducted linear regressions for difficulty describing feelings at each n-back level (Fig. 1, right). Consistent with prior work linking WM and alexithymia (Frawley and Smith, 2001, Kano and Fukudo, 2013, Vermeulen et al., 2018), we observed that lower d-prime values correlated with higher alexithymia during the 3-back condition, when WM demands were greatest.
Fig. 1.
Working memory (WM) performance correlated with a facet of alexithymia. i) Task performance (d-prime) decreased as WM-load (n-back level) increased. Tukey-corrected t-tests indicated that d-prime values differed across each condition (2- vs. 1-back: t[161]=-13.5, p < 0.001; 2- vs. 3-back: t[161]=8.5, p < 0.001; 3- vs. 1-back: t[161]=-20.0, p < 0.001). ii) Performance negatively correlated with difficulty describing feelings (DDF, one facet of alexithymia) during the 3-back (i.e., the highest WM-load condition), but not the 1- or 2-back conditions (covariates: age, sex, and ethnicity). e = estimate std.
A whole-brain ANOVA identified WM-load effects notably in lPFC, dmPFC, parietal cortices, medial (m)PFC, PCC, and STG clusters (Table S1). To visualize WM-related (de)activations, β-maps from the 3-back versus 0-back contrast are shown in Fig. 2. Consistent with prior work (Hu et al., 2013, Rocca et al., 2014), as WM-load increased, CEN regions (e.g., lPFC, dmPFC, parietal cortices) showed increased activations and DMN regions (e.g., mPFC, PCC, STG extending into supramarginal gyrus) showed increased deactivations.
Fig. 2.
WM-related brain activity correlated with a facet of alexithymia. A, B) Task-related activations in the lPFC and dmPFC (β-values) increased as WM-load increased. Tukey-corrected t-tests indicated that lPFC β-values differed across each task condition (indicated by * in [i]; 2- vs. 1-back: t[148]=-17.6, p < 0.00; 2- vs. 3-back: t[148]=3.4, p < 0.01; 3- vs. 1-back: t[148]=-16.9, p < 0.001) whereas dmPFC β-values did not differ for the 2- vs. 3-back conditions (2- vs. 1-back: t[148]=-18.0, p < 0.001; 2- vs. 3-back: t[148]=0.9, p = 0.66; 3- vs. 1-back: t[148]=-19.0, p < 0.001. lPFC and dmPFC (β-values) negatively correlated with difficulty describing feelings (DDF). Specifically, less WM-related activations were linked with higher DDF values during high WM-load conditions (i.e., 2- and 3-back levels, but not 1-back; as indicated by solid best-fitting lines in [ii]). C, D) Task-related deactivations in the mPFC and STG increased as WM-load increased. Tukey-corrected t-tests indicated that mPFC β-values differed across each condition (2- vs. 1-back: t[148]=14.2, p < 0.00; 2- vs. 3-back: t[148]=-4.3, p < 0.001; 3- vs. 1-back: t[148]=11.6, p < 0.001) whereas STG β-values did not differ for the 2- vs. 3-back conditions (2- vs. 1-back: t[148]=13.3, p < 0.001; 2- vs. 3-back: t[148]=0.9, p = 0.33; 3- vs. 1-back: t[148]=14.2, p < 0.001). mPFC and STG β-values positively correlated with DDF. Specifically, less WM-related deactivations were linked with higher DDF values during the highest WM-load condition (i.e., 3-back level). STG deactivations at the 1-back level also positively correlated with DDF. e = estimate std.
3.2. Alexithymia-related outcomes
Regarding WM-related task performance activations, lPFC and dmPFC β-values negatively correlated with difficulty describing feelings at the 2- and 3-back levels, but not at the 1-back level (Fig. 2A-B, right). Less lPFC and dmPFC activations when WM demands were high was linked with greater alexithymia. Speaking to regional specificity, similar relations were not observed when considering other regions displaying WM-related task performance activations (i.e., bilateral parietal cortices; see Figure S1A).
Regarding task-related deactivations, mPFC and STG β-values positively correlated with difficulty describing feelings at the 3-back level (Fig. 2C-D, right). Less mPFC and STG deactivations when WM demands were greatest was linked with more alexithymia. A similar association was also observed at the 1-back level for the STG. Speaking to regional specificity, similar associations were not observed when considering another region displaying task-related deactivations, the PCC (Figure S1B). Given prior work linking alexithymia with lPFC and STG activity (Kano and Fukudo, 2013, Nadeau, 2021), these regions became the focus of subsequent analyses.
3.3. SU-related outcomes
Given that behavior and brain measures from the 3-back condition showed the most robust associations with difficulty describing feelings, we focused on variables from this task level in subsequent analyses. We considered descriptive statistics and correlations across WM brain activity, WM-related task performance, self-reported alexithymia, and self-reported SU variables (Table 1).
Table 1.
Descriptive statistics and correlations across variables.
| M | SD | Pearson Correlations |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | ||
|
14.90 | 0.69 | − | ||||||||||||
|
0.52 | 0.50 | 0.03 | − | |||||||||||
|
0.85 | 0.36 | 0.11 | −0.10 | − | ||||||||||
|
0.38 | 0.49 | 0.09 | −0.00 |
0.16 * |
− | |||||||||
|
0.20 | 0.40 |
0.18 * |
0.08 | 0.09 | 0.49*** | − | ||||||||
|
0.32 | 0.47 | 0.23** | −0.03 | 0.15† | 0.31*** | 0.21** | − | |||||||
|
0.50 | 0.23 | 0.13 | 0.05 | 0.08 | −0.07 | −0.02 | −0.13 | − | ||||||
|
−0.33 | 0.23 | −0.03 | −0.06 | −0.09 | −0.08 | −0.02 | 0.01 | −0.08 | − | |||||
|
0.60 | 0.18 | 0.08 | −0.03 | 0.05 | −0.02 | −0.07 | −0.03 | 0.31*** | −0.27*** | − | ||||
|
12.62 | 4.88 | 0.07 | −0.23** | 0.14† | 0.02 | 0.05 |
0.16 * |
−0.17 * |
0.18 * |
−0.18 * |
− | |||
|
9.55 | 37.91 | 0.04 | 0.08 | −0.09 |
0.19 * |
0.29*** | 0.01 | −0.03 | −0.03 | −0.11 | 0.23** | − | ||
|
13.09 | 55.43 | 0.15† | 0.12 | 0.06 | 0.27*** | 0.44*** | 0.14 | 0.03 | −0.05 | −0.03 | 0.09 | 0.48*** | − | |
|
4.22 | 20.37 | 0.13 | −0.05 | 0.08 | 0.06 | 0.09 | 0.13 | 0.12 | 0.02 | 0.02 | 0.04 | 0.04 | 0.24** | − |
Note.
*p < 0.05; **p < 0.01; ***p < 0.001; †p = marginal; W1a=Wave 1, Visit 1; W1b=Wave 1, Visit 2; W2 =Wave 2; a Female= 0 (51 %,n= 134), Male= 1 (49 %,n= 130); b non-Hispanic/Latino(a)= 0 (16 %,n= 41), Hispanic/Latino(a)= 1 (84 %,n= 223); c Non-User= 0 (67 %,n= 176), User= 1 (33 %,n= 88); d Non-User= 0 (82 %,n= 217), User= 1 (18 %,n= 47); e Non-User= 0 (68 %,n= 179), User= 1 (32 %,n= 85); sample information based on larger project’s W1a sample size (N = 264). lPFC activations and STG deactivations are at the 3-back level. Task performance = d-prime at the 3-back level.
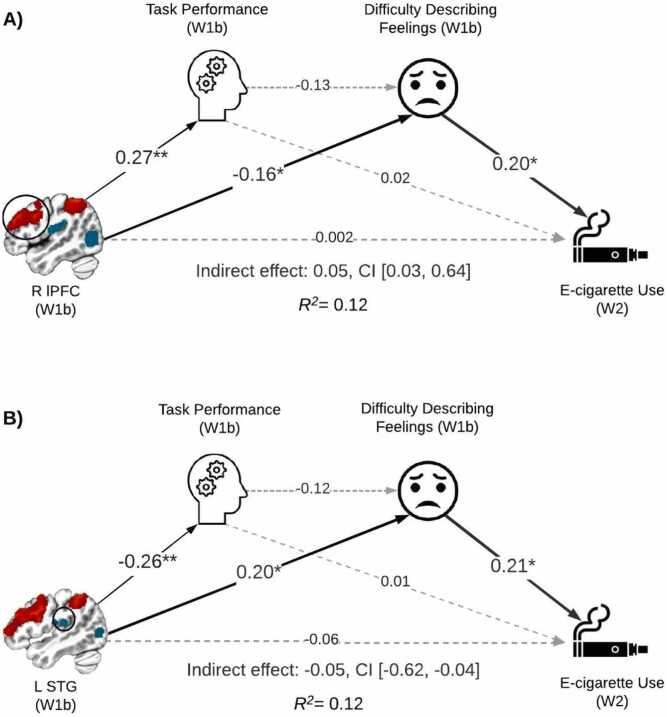
Across the six models linking WM brain activity (2: lPFC activations, STG deactivations), WM-related task performance, alexithymia, and SU outcomes (3: e-cigarette, cannabis, alcohol), there was no support for serial mediation. However, within the two e-cigarette models, there was support for simple mediation via difficulty describing feelings (Table 2, Fig. 3). That is, less WM-related lPFC activation was associated with higher alexithymia scores, and higher alexithymia predicted more e-cigarette use days (indirect effect: 0.05, CI[0.03,0.64], Fig. 3A). Similarly, less STG deactivation was associated with higher alexithymia which, in turn, predicted more e-cigarette use (indirect effect: −0.05, CI[-0.62,-0.04], Fig. 3B). While lPFC activations and STG deactivations both linked with performance, the association between d-prime and difficulty describing feelings was not significant, suggesting that the brain variables accounted for the shared variance with alexithymia (i.e., task performance did not uniquely contribute to alexithymia). Noteworthy, the direct effects of lPFC and STG (de)activations on e-cigarette use were not significant. Similar simple mediation effects were not observed when considering cannabis and alcohol use (Tables S2 and S3) as the paths between alexithymia and use failed to reach significance. For the e-cigarette models, observed associations remained unchanged when including mean FD as an additional covariate (Table S4).
Table 2.
Serial mediation outcomes linking brain, alexithymia, and e-cigarette use.
| Task Performance W1b (M1) | Difficulty Describing Feelings W1b (M2) | E-cigarette Use W2 (Y) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| lPFC (activation) | Coefficient | (95 % CI) | SE | z Value | Coefficient | (95 % CI) | SE | z Value | Coefficient | (95 % CI) | SE | z Value |
| Intercept | 3.14 | (−0.02, 1.04) | 0.27 | 1.86 | 1.18 | (−10.36, 21.20) | 7.84 | 0.70 | −2.46 | (−177.15, 18.52) | 51.53 | −1.38 |
| Age | 0.01 | (−0.03, 0.04) | 0.02 | 0.10 | 0.11 | (−0.25, 1.70) | 0.50 | 1.49 | 0.09 | (−2.76, 11.32) | 3.54 | 1.09 |
| Sexa | −0.09 | (−0.08, 0.02) | 0.03 | −1.11 | −1.17* | (−3.10, 0.04) | 0.77 | −1.99 | 0.12 | (−2.58, 19.36) | 5.54 | 1.26 |
| Ethnicityb | −0.03 | (−0.11, 0.08) | 0.05 | −0.33 | 0.11 | (−0.62, 3.69) | 1.07 | 1.33 | −0.08 | (−20.63, 4.08) | 6.46 | −0.99 |
| Lifetime E-Cig. Use (W1a) | 0.01 | (−0.06, 0.07) | 0.03 | 0.08 | −0.07 | (−2.32, 0.75) | 0.78 | −0.89 | 0.25* | (3.74, 28.45) | 6.47 | 2.34 |
| R lPFC (W1b) | 0.27** | (0.05, 0.35) | 0.07 | 2.77 | −0.16* | (−7.09, −0.11) | 1.73 | −2.00 | 0.002 | (−20.51, 18.62) | 9.84 | 0.02 |
| Task Performance (W1b) | - | - | - | - | −0.13 | (−8.70, 1.23) | 2.50 | −1.49 | 0.02 | (−21.79, 25.69) | 11.67 | 0.34 |
| Difficulty Describing Feelings (W1b) | - | - | - | - | - | - | - | - | 0.20* | (0.22, 2.54) | 0.60 | 2.05 |
| R2= 0.08 | R2= 0.11 | R2= 0.12 | ||||||||||
| STG (deactivation) | Task Performance W1b (M1) | Difficulty Describing Feelings W1b (M2) | E-cigarette Use W2 (Y) | |||||||||
| Intercept | 2.86 | (−0.15, 1.02) | 0.29 | 1.62 | 1.35 | (−7.83, 19.88) | 7.51 | 0.83 | −2.47 | (−171.30, 25.25) | 50.82 | −1.41 |
| Age | 0.03 | (−0.03, 0.05) | 0.02 | 0.40 | 0.10 | (−0.22, 1.68) | 0.50 | 1.34 | 0.09 | (−3.28, 10.45) | 3.42 | 1.08 |
| Sexa | −0.10 | (−0.09, 0.03) | 0.03 | −1.14 | −0.16† | (−2.99, 0.10) | 0.78 | −1.89 | 0.12 | (−2.17, 20.01) | 5.71 | 1.17 |
| Ethnicityb | −0.03 | (−0.09, 0.08) | 0.05 | −0.26 | 0.11 | (−0.84, 3.61) | 1.14 | 1.23 | −0.08 | (−21.97, 3.62) | 6.75 | −1.00 |
| Lifetime E-Cig. Use (W1a) | −0.03 | (−0.07, 0.05) | 0.03 | −0.30 | −0.05 | (−2.08, 1.08) | 0.79 | −0.64 | 0.25* | (4.14, 28.60) | 6.42 | 2.34 |
| L STG (W1b) | −0.26** | (−0.32, −0.05) | 0.06 | −2.84 | 0.20* | (0.73, 7.85) | 1.78 | 2.17 | −0.06 | (−24.01, 8.62) | 8.15 | −0.89 |
| Task Performance (W1b) | - | - | - | - | −0.12 | (−8.55, 1.03) | 2.39 | −1.47 | 0.01 | (−22.61, 25.82) | 11.87 | 0.14 |
| Difficulty Describing Feelings (W1b) | - | - | - | - | - | - | - | - | 0.21* | (0.28, 2.71) | 0.63 | 2.07 |
| R2= 0.07 | R2= 0.12 | R2= 0.12 | ||||||||||
Note. *p < 0.05, **p < 0.01, †p = marginal; W1a = Wave 1, Visit 1; W1b = Wave 1, Visit 2; W2 = Wave 2; a Female= 0, Male= 1; b non-Hispanic/Latino(a)= 0, Hispanic/Latino(a)= 1. Coefficients represent standardized effects. See also Fig. 3. lPFC activations and STG deactivations are at the 3-back level. Task performance = d-prime at the 3-back level.
Fig. 3.
Serial mediation models linking brain, alexithymia, and e-cigarette use. Simple mediation linking brain activity and e-cigarette use via difficulty describing feelings was observed. A) Less WM-related lPFC activation was associated with higher alexithymia scores and higher alexithymia predicted more e-cigarette use days. B) Similarly, less WM-related STG deactivation was associated with higher alexithymia which, in turn, predicted more use days. *p < 0.05, **p < 0.01; W1b = Wave 1, Visit 2; W2 = Wave 2. Covariates were included in the model, but are not depicted (i.e., age, biological sex, ethnicity, and lifetime e-cigarette use). Coefficients represent standardized effects. See also Table 2.
3.4. E-cigarette use expectancies
When considering e-cigarette use expectancies, we again observed support for simple mediation via difficulty describing feelings (Figure S2, Table S5). Less lPFC activation was associated with higher alexithymia, and in turn alexithymia predicted more positive outcome expectancies for e-cigarette use (indirect effect: 0.07, CI[0.0001,0.02], Figure S2A). Less STG deactivation was also associated with higher alexithymia which predicted more positive outcome expectancies (indirect effect: −0.06, CI[-0.02,-0.0001], Figure S2B).
4. Discussion
We examined interrelations between WM-related brain activity, WM-related task performance, and alexithymia characteristics as mechanistic paths to teen SU. Consistent with prior adult studies (Hu et al., 2013, Rocca et al., 2014), as WM-load increased, we observed decreased performance accompanied by increased lPFC activations and STG deactivations in our adolescent sample. Regarding alexithymia outcomes, we observed that greater difficulty describing feelings correlated with reduced WM-related task performance, and less lPFC activations and STG deactivations. As alexithymia’s potential cognitive manifestations are underappreciated (Riadh et al., 2019), especially during adolescence, these observations add to growing evidence highlighting alterations in WM abilities and PFC activity as contributors to emotional processing difficulties (Frawley and Smith, 2001). As adolescence is a development period characterized by ongoing PFC maturation, reduced PFC activity linked with the cognitive processing of emotions could place some individual at greater risk for affect dysregulation and/or SU. Notably, in a serial mediation framework we observed that less lPFC activation (and STG deactivation) was linked with higher alexithymia, which predicted more e-cigarette use days. Providing insight into why some adolescents may use, we observed that less lPFC activation (and STG deactivation) was linked with higher alexithymia which predicted more positive outcome expectancies of e-cigarette use.
As expected, we observed WM-load effects manifesting as increased activations across CEN regions (e.g., lPFC, dmPFC, and parietal cortices), which are commonly engaged during attention-demanding tasks (Fox et al., 2005). As primary CEN nodes, lPFC activity often positively correlates with cognitive performance (Hu et al., 2013) and is also involved with affective processes (Koenigs and Grafman, 2009); for example, emotion regulation through reappraisal (Golkar et al., 2012). Consistent with this neurobiological-cognitive-affective intersection, WM training has been explored as an intervention to modulate CEN function and augment emotion regulation capacities (Cui et al., 2024, Xiu et al., 2016, Zhang et al., 2024). Additionally, we observed WM-load effects manifesting as increased deactivations across DMN regions (e.g., STG, mPFC, PCC). On the one hand, DMN regions show increased activations during self-referential, social cognition, and drug cue-reactivity paradigms (Greicius et al., 2003, Hill-Bowen et al., 2021, Qin and Northoff, 2011, Wilcox et al., 2016). Conversely, insufficient DMN deactivation during other cognitive tasks is linked with reduced performance (Hu et al., 2013, Rocca et al., 2014) suggesting the misallocation of processing resources away from external stimuli towards internally-oriented information (Flannery et al., 2022, Lerman et al., 2014, Sutherland and Stein, 2018). A reduced capacity to deactivate DMN regions may contribute to multiple neuropsychiatric disorders (Hu et al., 2013, Rocca et al., 2014), including nicotine addiction (Loughead et al., 2015, Sutherland et al., 2012, Sutherland et al., 2015). Consistent with these intersections, mindfulness meditation has been explored as an intervention to modulate DMN function. Such DMN modulation can be accompanied by increased psychological well-being including augmented emotion regulation, interoceptive awareness, attentional control, executive functioning (Bauer et al., 2019, Brewer et al., 2011), and reduced drug cravings (Brewer et al., 2013, Lorenzetti et al., 2023).
As hypothesized, we observed that more alexithymia characteristics correlated with reduced WM-related task performance and, highlighting neurobiological loci, with reduced lPFC activations and STG deactivations. Speaking to regional specificity, such brain-behavior relations were not observed when considering other CEN (i.e., parietal cortices) or DMN regions (i.e., PCC; see supplement). The lPFC and STG have been linked with alexithymia in the context of affective processing, but less so when considering cognitive processes (Bigler et al., 2007, Correro et al., 2021, Moriguchi et al., 2007, Zevin, 2009). Our observations align with the emerging perspective that alexithymia relates to altered representations of emotions in WM (Frawley and Smith, 2001, Koven and Thomas, 2010, Luminet et al., 2021) limiting one’s inference abilities about the affective state of self or others (Morra et al., 2011). From a developmental perspective, WM capacity improves throughout adolescence (Ahmed et al., 2022, Andre et al., 2015). Such limitations in WM among adolescents may negatively impact integration of emotions and increase alexithymia. To the extent that lPFC is involved in emotion-related experiential processes and their cognitive processing (Bermond et al., 2007, Correro et al., 2021, Moriguchi et al., 2007), we speculate that reduced lPFC function, perhaps due to the normative maturational-delay of PFC regions, may contribute to less use of regulation strategies among adolescents higher in alexithymia (Laloyaux et al., 2015). Furthermore, the STG contributes to social cognition, emotional understanding, and self-referential processes (Bigler et al., 2007, Brooks and Stein, 2016, Sajonz et al., 2010, Yıldırım Ayaz and Dincer, 2021), each of which may contribute to difficulties describing feelings. As such, altered lPFC and/or STG functioning may, in part, underlie affective and cognitive manifestations linked with greater alexithymia characteristics.
Critically, we delineated neurobiological and affective factors leading to e-cigarette use. We observed that difficulty describing feelings mediated the association between WM-related brain activity (i.e., lPFC, STG) and subsequent e-cigarette use. Noteworthy, although WM-related task performance correlated with alexithymia, the direct effect was not significant when accounting for WM-related brain activity and the covariates. These findings highlight the value of considering brain activity (as opposed to only task performance) when considering alexithymia’s potential role in adolescent SU, in particular how lPFC and STG activity may contribute to difficulties describing feelings. Our outcomes align with prior work noting that brain measures (e.g., PFC activity) influence individual differences in WM among children above and beyond task performance (Rosenberg et al., 2020). Further, our findings suggest that both WM-related task performance and alexithymia levels vary as a function of brain activity, specifically in the lPFC (activations) and STG (deactivations). Our observations extend prior alexithymia work by implicating specific regional brain activity that may account for previous observations linking behavioral measures of executive functioning with alexithymia (Frawley and Smith, 2001). In other words, our outcomes offer a neurobiological account of factors linked with alexithymia (i.e., altered lPFC and STG function) which complements and enriches extant psychological accounts (i.e., altered executive functioning; Frawley and Smith, 2001; Luminet et al., 2021).
Additionally, although some data suggest a direct WM-to-SU link among adolescents (Khurana et al., 2017, Tarter et al., 2003), other psychological factors may account for this association (Giancola et al., 2001, Grenard et al., 2008). Indeed, we did not observe a direct effect between WM-related brain activity and e-cigarette use nor a significant path between WM-related task performance and e-cigarette use. SU behaviors are complex and stem from a wide array of biological, psychological and social factors (Volkow and Blanco, 2023). As such, mediators can stand in between the link of WM to SU. Here, we show that alterations in WM-related brain activity contribute indirectly to adolescent e-cigarette use via alexithymia. In contrast, while alexithymia may contribute to alcohol and cannabis use (Cruise and Becerra, 2018, Dorard et al., 2008, Kajanoja et al., 2019), we did not observe interrelations between difficulty describing feelings and use of these substances.
Difficulty identifying feelings seems to be more strongly associated with cannabis and alcohol use among teenagers compared to difficulty describing feelings (Dorard et al., 2017, Gatta et al., 2014). Importantly, our prior work demonstrated that difficulty describing feelings (but not identifying) predicted adolescent e-cigarette use (Sutherland, Fallah-Sohy, et al., 2022). Perhaps adolescents with difficulty identifying feelings use substances that “numb out” distress such as cannabis and alcohol compared to using nicotine. Nicotine, found in e-cigarettes, enhances cognition and promotes subjective feeling of reduced stress, increased focus, and improved mood for a short period of time (Valentine and Sofuoglu, 2018). As such, nicotine may enhance processing and/or verbalization of emotions (Sutherland, Fallah-Sohy, et al., 2022). Adolescents with difficulty describing feelings may be more prone to use e-cigarettes given that nicotine’s effects are more immediate and predictable compared to marijuana and alcohol which also have more variable effects on emotion regulation and cognition which can also be context-related (Moskal et al., 2023, Petit et al., 2015, Zimmermann et al., 2017). Moreover, e-cigarettes are easy to obtain, are viewed by adolescents as being safer and more socially acceptable (Kong et al., 2019) and, as such, teens may vape to connect emotionally with others. Adolescents with less WM-related brain activity may be more prone to experience difficulties communicating emotions and view e-cigarette use as a viable coping strategy. Ancillary serial mediation analyses supported this interpretation. Difficulty describing feelings mediated the association between WM-related brain activity and positive outcome expectancies for e-cigarette use, suggesting that teens higher in alexithymia view e-cigarette use as a way to alleviate negative feelings. Higher alexithymia characteristics are also indicative of lower emotional intelligence (Fossum and Montoya, 2023, Kano and Fukudo, 2013) which relates to increased risk-taking (Kano and Fukudo, 2013), and susceptibility to impulsive SU-decisions (Kopera et al., 2020, Wilcox et al., 2016).
4.1. Implications
Our findings highlight intervention targets for adolescent e-cigarette use. Some data suggest that WM training, which induces changes in PFC regions enhancing neural efficiency (Thompson et al., 2016), may offer transferable benefits for the enhancement of affect regulation, reduction of internalizing symptoms, and less reliance on maladaptive coping strategies (Cui et al., 2024, Samimi et al., 2017, Wang et al., 2019). For example, WM training on a dual n-back task led to improved cognitive reappraisal among people with high anxiety potentially augmenting emotional regulation (Wang et al., 2019). Additionally, mindfulness meditation, which can shift activity patterns within and between CEN and DMN regions including the lPFC and STG, respectively (Tomasino and Fabbro, 2016, Zhou et al., 2020), can reduce ruminations and mind wandering while enhancing attentional control and self-monitoring. As such, mindfulness meditation could augment WM and regulation abilities (Bauer et al., 2019, Brewer et al., 2011) with implications for reducing SU (Brewer et al., 2013, Lorenzetti et al., 2023). For example, Dialectical Behavior Therapy for Adolescents (DBT-A; Fleischhaker et al., 2011), utilizing mindfulness and regulation techniques, holds promise as a SU treatment for those with high alexithymia characteristics (Salles et al., 2023). That is, DBT-A views impulsive behaviors, including SU, as learned behaviors that function as a means to regulate emotions. Accordingly, a primary focus of DBT-A is to help youth identify emotional triggers leading to SU and how to manage intense emotions more effectively without resorting to SU. Because conventional psychotherapy involving the exploration and articulation of feelings may be challenging for people higher in alexithymia, an alternative strategy may involve expressive writing, particularly for adolescents (Baikie and Wilhelm, 2005). Writing about emotional events has shown positive effects in terms of mental health outcomes particularly for those with difficulties describing feelings (Baikie, 2008). Such interventions may be less stigmatizing, cheaper, and more easily implemented than e-cigarette-focused interventions.
4.2. Limitations
First, although alterations in WM-related brain activity may precede alexithymia (Nadeau, 2021, Wilcox et al., 2016), bidirectional relations may exist (Koven and Thomas, 2010). Future work including an additional timepoint to account for temporal precedence is crucial.1 Second, our findings may not generalize to all adolescents as our sample was predominantly Hispanic/Latino(a) youth who can be at increased risk for internalizing disorders (Anderson and Mayes, 2010) and e-cigarette use relative to other ethnicities (Lanza et al., 2017). Third, we used self-report measures which are susceptible to biased perceptions (Betka et al., 2018). Similarly, stigma or misunderstanding of questions could have impacted SU reports (Brener et al., 2003). Further, we did not assess recent SU which can alter brain activity and cognitive functioning (Vik et al., 2004) prior to MRI scans. Fourth, although we statistically controlled for baseline SU, assessing youth before the onset of any use remains important for large, multisite studies (Casey et al., 2018). This could disentangle nuances between onset and maintenance of use as our findings could be interpreted as initiation, maintenance, or escalation of use. Lastly, our correlations showed that females reported higher levels of alexithymia, future work should consider examining differences across biological sex.
4.3. Conclusion
This study elucidated interrelations between WM abilities, alexithymia, and adolescent e-cigarette use in a serial mediation framework. Adolescents with reduced WM-related brain activity (lPFC activations, STG deactivations) reported greater difficulty describing feelings, and, in turn more days of e-cigarette use. Interventions targeting WM abilities, affective regulation, and verbalization of emotions may provide utility in reducing adolescent e-cigarette use.
Role of funding sources
This work was supported by the National Institutes of Health (U54MD012393 [Sub-Project:5378] mPIs: Trucco, Sutherland; T32DA043449 Director: Gonzalez) and the Florida Department of Health (24K09 PI: Trucco). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Florida Department of Health.
CRediT authorship contribution statement
Sutherland Benjelene D: Writing – review & editing, Writing – original draft, Visualization, Validation, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Lauren D. Hill-Bowen: Writing – review & editing, Formal analysis, Data curation. Elisa M. Trucco: Writing – review & editing, Supervision, Resources, Investigation, Funding acquisition, Conceptualization. Angela R. Laird: Writing – review & editing, Resources, Data curation. Matthew T. Sutherland: Writing – review & editing, Writing – original draft, Supervision, Resources, Methodology, Funding acquisition, Conceptualization.
Declaration of Competing Interest
All authors declare that they have no conflicts of interest.
Acknowledgements
National Institute on Drug Abuse Grant/Award Number: T32DA043449, National Institute on Minority and Health Disparities Grant/Award Numbers: U54MD012393, ID: 5378, 3U54 MD012393–04S2. We thank family participation in the Antecedents and Consequences of Electronic Nicotine Delivery Systems (ACE) Project, scanner techs, and ACE staff.
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2025.101634.
We also conducted post-hoc serial mediation analyses to clarify possible bidirectional associations between WM-related task performance and alexithymia. Specifically, we assessed whether alexithymia (M1: difficulty describing feelings) and/or WM-related task performance (M2: d-prime) mediated the impact of WM-related lateral PFC activations (X) on e-cigarette use (Y). In other words, we “swapped the order” of the two mediators. We observed that lateral PFC activity was linked with alexithymia (estimate = −4.22, p = 0.02), however alexithymia did not link with WM-related task performance (estimate = −0.01, p = 0.15) nor did WM-related task performance predict days of e-cigarette use (estimate = 3.91, p = 0.73) in this model. Given these null outcomes, which did not support serial mediation (indirect effect = 0.07, CI [-0.46, 0.92]), we retained our original model. We performed a similar post-hoc analysis when considering STG deactivations and observed the same outcomes. That is, STG deactivations were linked with alexithymia (M1; estimate = 4.49, p = 0.01), however alexithymia did not link with WM-related task performance (M2; estimate = −0.004, p = 0.16) nor did task performance predict e-cigarette use (Y; estimate = 1.64, p = 0.89). In other words, support for serial mediation was not detected (indirect effect = −0.03, CI [-0.82, 0.59]). Notably, alexithymia was not a predictor of WM-related task performance in either model which we viewed as supportive of our original models’ directionality/ordering of mediators.
Appendix A. Supplementary material
Supplementary material
Data availability
Data will be made available on request.
References
- Ahmed S.F., Ellis A., Ward K.P., Chaku N., Davis-Kean P.E. Working memory development from early childhood to adolescence using two nationally representative samples. Dev. Psychol. 2022;58(10):1962–1973. doi: 10.1037/dev0001396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E.R., Mayes L.C. Race/ethnicity and internalizing disorders in youth: a review. Clin. Psychol. Rev. 2010;30:338–348. doi: 10.1016/j.cpr.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Andre J., Picchioni M., Zhang R., Toulopoulou T. Working memory circuit as a function of increasing age in healthy adolescence: a systematic review and meta-analyses. Neuroimage Clin. 2015;11(12):940–948. doi: 10.1016/j.nicl.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby R.M., Parker J.D.A., Taylor G.J. The twenty-item toronto alexithymia scale: I. Item selection and cross-validation of the factor structure. J. Psychosom. Res. 1994;38(1):23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Baikie K.A. Who does expressive writing work for? Examination of alexithymia, splitting, and repressive coping style as moderators of the expressive writing paradigm. Br. J. Health Psychol. 2008;13(1):61–66. doi: 10.1348/135910707x250893. [DOI] [PubMed] [Google Scholar]
- Baikie K.A., Wilhelm K. Emotional and physical health benefits of expressive writing. Adv. Psychiatr. Treat. 2005;11(5):338–346. doi: 10.1192/apt.11.5.338. [DOI] [Google Scholar]
- Bauer C.C.C., Whitfield-Gabrieli S., Díaz J.L., Pasaye E.H., Barrios F.A. From State-to-Trait meditation: reconfiguration of central executive and default mode networks. eneuro. 2019;6(6) doi: 10.1523/eneuro.0335-18.2019. ENEURO.0335-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermond B., Vorst H.C.M., Moormann P.P. Cognitive neuropsychology of alexithymia: implications for personality typology. Cogn. Neurospychiatry. 2007;11(3) doi: 10.1080/13546800500368607. [DOI] [PubMed] [Google Scholar]
- Betka S., Pfeifer G., Garfinkel S., Prins H., Bond R., Sequeira H., Duka T., Critchley H. How do Self-Assessment of alexithymia and sensitivity to bodily sensations relate to alcohol consumption? Alcohol. Clin. Exp. Res. 2018;42(1):81–88. doi: 10.1111/acer.13542. [DOI] [PubMed] [Google Scholar]
- Bigler E.D., Mortensen S., Neeley E.S., Ozonoff S., Krasny L., Johnson M., Lu J., Provencal S.L., McMahon W., Lainhart J.E. Superior temporal gyrus, language function, and autism. Dev. Neuropsychol. 2007;31(2):217–238. doi: 10.1080/87565640701190841. [DOI] [PubMed] [Google Scholar]
- Brener N.D., Billy J.O.G., Grady W.R. Assessment of factors affecting the validity of self-reported health-risk behavior among adolescents: evidence from the scientific literature. J. Adolesc. Health. 2003;33(6):436–457. doi: 10.1016/s1054-139x(03)00052-1. [DOI] [PubMed] [Google Scholar]
- Brewer J.A., Worhunsky P.D., Gray J.R., Tang Y.-Y., Weber J., Kober H. Meditation experience is associated with differences in default mode network activity and connectivity. Proc. Natl. Acad. Sci. 2011;108(50):20254–20259. doi: 10.1073/pnas.1112029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer J.A., Garrison K.A., Whitfield-Gabrieli S. What about the “Self” is processed in the posterior cingulate cortex? Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S.J., Stein D.J. Chapter 54 - brain volumes in adolescents with alcohol use disorder. Neuropathol. Drug Addict. Subst. Misuse. 2016;1:587–599. doi: 10.1016/B978-0-12-800213-1.00054-7. [DOI] [Google Scholar]
- Casey B.J., Getz S., Galvan A. The adolescent brain. Dev. Rev. 2008;28:62–77. doi: 10.1016/j.dr.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Cannonier T., Conley M.I., Cohen A.O., Barch D.M., Heitzeg M.M., Soules M.E., Teslovich T., Dellarco D.V., Garavan H., Orr C.A., Wager T.D., Banich M.T., Speer N.K., Sutherland M.T., Riedel M.C., Dick A.S., Bjork J.M., Thomas K.M., Chaarani B., Mejia M.H., Hagler D.J.J., Daniela Cornejo M., Sicat C.S., Harms M.P., Dosenbach N.U.F., Rosenberg M., Earl E., Bartsch H., Watts R., Polimeni J.R., Kuperman J.M., Fair D.A., Dale A.M., & Workgroup., A. I. A The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018;32:43–54. doi: 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correro A.N., Paitel E.R., Byers S.J., Nielson K.A. The role of alexithymia in memory and executive functioning across the lifespan. Cogn. Emot. 2021;35(3):524–539. doi: 10.1080/02699931.2019.1659232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W., Hyde J.S. Software tools for analysis and visualization of fMRI data. NMR Biomed. 1997;10:171–178. doi: 10.1002/(SICI)1099-1492(199706/08)10:4/5%3C171::AID-NBM453%3E3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Cristello J.V., Sutherland M.T., Trucco E.M. A preliminary validation of the adolescent e-cigarette consequences questionnaire. Drug Alcohol Depend. 2020;213 doi: 10.1016/j.drugalcdep.2020.108118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruise K.E., Becerra R. Alexithymia and problematic alcohol use: a critical update. Addict. Behav. 2018;77:232–246. doi: 10.1016/j.addbeh.2017.09.025. [DOI] [PubMed] [Google Scholar]
- Cui X., Zhang S., Yu S., Ding Q., Li X. Does working memory training improve emotion regulation and reduce internalizing symptoms? A pair of three-level meta-analyses. Behav. Res. Ther. 2024;179 doi: 10.1016/j.brat.2024.104549. [DOI] [PubMed] [Google Scholar]
- Dai H., Mei L., Minjun M., Xiaofei S. Regional homogeneity of intrinsic brain activity related to the main alexithymia dimensions. Gen. Psychiatry. 2018;31(1) doi: 10.1136/gpsych-2018-000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly M., Egan M., Quigley J., Delaney L., Baumeister R.F. Childhood self-control predicts smoking throughout life: evidence from 21,000 cohort study participants. Health Psychol. 2016;35(11):1254–1263. doi: 10.1037/hea0000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorard G., Berthoz S., Phan O., Corcos M., Bungener C. Affect dysregulation in cannabis abusers. Eur. Child Adolesc. Psychiatry. 2008;17(5):274–282. doi: 10.1007/s00787-007-0663-7. [DOI] [PubMed] [Google Scholar]
- Dorard G., Bungener C., Phan O., Edel Y., Corcos M., Berthoz S. Is alexithymia related to cannabis use disorder? Results from a case-control study in outpatient adolescent cannabis abusers. J. Psychosom. Res. 2017;95:74–80. doi: 10.1016/j.jpsychores.2017.02.012. [DOI] [PubMed] [Google Scholar]
- Duckworth A.L., Steinberg L. Unpacking Self-Control. Child Dev. Perspect. 2015;9(1):32–37. doi: 10.1111/cdep.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O., Birman D., Schaer M., Koyejo O.O., Poldrack R.A., Gorgolewski K.J. MRIQC: advancing the automatic prediction of image quality in MRI from unseen sites. Plos One. 2017;12(9) doi: 10.1371/journal.pone.0184661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban O., Blair R., Markiewicz C.J., Berleant S.L., Moodie C., Ma F., Isik A.I., et al. Zenodo; 2018. fmriprep. in software. [Google Scholar]
- Esteban O., Markiewicz C.J., Blair R.W., Moodie C.A., Isik A.I., Erramuzpe A., Kent J.D., Goncalves M., Dupre E., Snyder M., Oya H., Ghosh S.S., Wright J., Durnez J., Poldrack R.A., Gorgolewski K.J. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods. 2019;16(1):111–116. doi: 10.1038/s41592-018-0235-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney K.E., Kampman K.M. Adverse effects of marijuana use. Cathol. Med. Assoc. 2016;83(2):174–178. doi: 10.1080/00243639.2016.1175707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery J.S., Riedel M.C., Hill-Bowen L.D., Poudel R., Bottenhorn K.L., Salo T., Laird A.R., Gonzalez R., Sutherland M.T. Altered large-scale brain network interactions associated with HIV infection and error processing. Netw. Neurosci. 2022;6(3):791–815. doi: 10.1162/netn_a_00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischhaker C., Böhme R., Sixt B., Brück C., Schneider C., Schulz E. Dialectical behavioral therapy for adolescents (DBT-A): a clinical trial for patients with suicidal and self-injurious behavior and borderline symptoms with a one-year Follow-up. Child Adolesc. Psychiatry Ment. Health. 2011;5(1):3. doi: 10.1186/1753-2000-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossum J.L., Montoya A.K. When to use different inferential methods for power analysis and data analysis for between-subjects mediation. Adv. Methods Pract. Psychol. Sci. 2023;6(2) doi: 10.31234/osf.io/5tm2x. [DOI] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frawley W., Smith R.N. A processing theory of alexithymia. Cogn. Syst. Res. 2001;2(3):189–206. doi: 10.1016/S1389-0417(01)00029-8. [DOI] [Google Scholar]
- Gatta M., Facca I., Colombo E., Svanellini L., Montagnese S., Schiff S. Alexithymia, psychopathology and alcohol misuse in adolescence: a population based study on 3556 teenagers. Neurosci. Med. 2014;05(01):60–71. doi: 10.4236/nm.2014.51009. [DOI] [Google Scholar]
- George O., Koob G.F. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci. amp Biobehav. Rev. 2010;35(2):232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancola P.R., Shoal G.D., Mezzich A.C. Constructive thinking, executive functioning, antisocial behavior, and drug use involvement in adolescent females with a substance use disorder. Exp. Clin. Psychopharmacol. 2001;9(2):215–227. doi: 10.1037/1064-1297.9.2.215. [DOI] [PubMed] [Google Scholar]
- Golkar A., Lonsdorf T.B., Olsson A., Lindstrom K.M., Berrebi J., Fransson P., Schalling M., Ingvar M., Öhman A. Distinct contributions of the dorsolateral prefrontal and orbitofrontal cortex during emotion regulation. Plos One. 2012;7(11) doi: 10.1371/journal.pone.0048107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green R., Wolf B.J., Chen A., Kirkland A.E., Ferguson P.L., Browning B.D., Bryant B.E., Tomko R.L., Gray K.M., Mewton L., Squeglia L.M. Predictors of substance use initiation by early adolescence. Am. J. Psychiatry. 2024;181(5):423–433. doi: 10.1176/appi.ajp.20230882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenard J.L., Ames S.L., Wiers R.W., Thush C., Sussman S., Stacy A.W. Working memory capacity moderates the predictive effects of drug-related associations on substance use. Psychol. Addict. Behav. 2008;22(3):426–432. doi: 10.1037/0893-164X.22.3.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C., Pascual M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol. 2010;44(1):15–26. doi: 10.1016/j.alcohol.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Haatveit B.C., Sundet K., Hugdahl K., Ueland T., Melle I., Andreassen O.A. The validity of d prime as a working memory index: results from the “Bergen n-back task. J. Clin. Exp. Neuropsychol. 2010;32(8):871–880. doi: 10.1080/13803391003596421. [DOI] [PubMed] [Google Scholar]
- Hammond C.J., Mayes L.C., Potenza M.N. Neurobiology of adolescent substance use and addictive behaviors: prevention and treatment implications. Adolesc. Med State Art. Rev. 2014;25(1):15–32. [PMC free article] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Thielke R., Payne L., Gonzalez N., Conde J.G. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O'Neal L., McLeod L., Delacqua G., Delasqua F., Kirby J., Duda S.N., & consortium, R. The REDCap consortium: building an international community of software partners. J. Biomed. Inf. 2019 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann S.A., Hayes T., Sutherland M.T., Trucco E.M. Risk factors for early use of e-cigarettes and alcohol: dimensions and profiles of temperament. Dev. Psychopathol. 2021:1–13. doi: 10.1017/s0954579421001565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A.J., Kassel J.D., Berbaum M., Mermelstein R. Adolescents’ expectancies for smoking to regulate affect predict smoking behavior and nicotine dependence over time. Drug Alcohol Depend. 2010;111(1-2):128–135. doi: 10.1016/j.drugalcdep.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill-Bowen L.D., Riedel M.C., Poudel R., Salo T., FLannery J.S., Camilleri J.A., Eickhoff S.B., Laird A.R., Sutherland M.T. The cue-reactivity paradigm: an ensemble of networks driving attention and cognition when viewing drug and natural reward-related stimuli. Neurosci. Behav. Rev. 2021;130:201–213. doi: 10.1016/j.neubiorev.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds O., Thompson T.W., Ghosh S.S., Yoo J.J., Whitfield-Gabrieli S., Triantafyllou C., Gabrieli J.D.E. Roles of default-mode network and supplementary motor area in human vigilance performance: evidence from real-time fMRI. J. Neurophysiol. 2013;109(5):1250–1258. doi: 10.1152/jn.00533.2011. [DOI] [PubMed] [Google Scholar]
- Hu Y., Chen X., Gu H., Yang Y. Resting-State glutamate and GABA concentrations predict Task-Induced deactivation in the default mode network. J. Neurosci. 2013;33(47):18566–18573. doi: 10.1523/jneurosci.1973-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland A., Ambrose B.K., Conway K.P., Borek N., Lambert E., Carusi C., Taylor K., Crosse S., Fong G.T., Cummings K.M., Abrams D., Pierce J.P., Sargent J., Messer K., Bansal-Travers M., Niaura R., Vallone D., Hammond D., Hilmi N., Kwan J., Piesse A., Kalton G., Lohr S., Pharris-Ciurej N., Castleman V., Green V.R., Tessman G., Kaufman A., Lawrence C., van Bemmel D.M., Heather L Kimmel H.L., Blount B., Yang L., O'Brien B., Tworek C., Alberding D., Hull L.C., Cheng Y., Maklan D., Backinger C.L., Compton W.M. Design and methods of the population assessment of tobacco and health (PATH) study. Tob. Control. 2016;26:371–378. doi: 10.1136/tobaccocontrol-2016-052934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajanoja J., Scheinin N.M., Karukivi M., Karlsson L., Karlsson H. Alcohol and tobacco use in men: the role of alexithymia and externally oriented thinking style. Am. J. Drug Alcohol Abus. 2019;45(2):199–207. doi: 10.1080/00952990.2018.1528267. [DOI] [PubMed] [Google Scholar]
- Kano M., Fukudo S. The alexithymic brain: the neural pathways linking alexithymia to physical disorders. Biopsychosoc. Med. 2013;7(1):1. doi: 10.1186/1751-0759-7-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M., Fukudo S., Gyoba J., Kamachi M., Tagawa M., Mochizuki H., Itoh M., Hongo M., Yanai K. Specific brain processing of facial expressions in people with alexithymia: an H215O-PET study. Brain. 2003;126(6):1474–1484. doi: 10.1093/brain/awg131. [DOI] [PubMed] [Google Scholar]
- Khurana A., Romer D., Betancourt L.M., Hurt H. Working memory ability and early drug use progression as predictors of adolescent substance use disorders. Addiction. 2017;112(7):1220–1228. doi: 10.1111/add.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M., Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behav. Brain Res. 2009;201(2):239–243. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn N., Eickhoff S.B., Scheller M., Laird A.R., Fox P.T., Habel U. Neural network of cognitive emotion regulation — an ALE meta-analysis and MACM analysis. NeuroImage. 2014;87:345–355. doi: 10.1016/j.neuroimage.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong G., Bold K.W., Morean M.E., Bhatti H., Camenga D.R., Jackson A., Krishnan-Sarin S. Appeal of JUUL among adolescents. Drug Alcohol Depend. 2019;205 doi: 10.1016/j.drugalcdep.2019.107691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopera M., Jakubczyk A., Suszek H., Glass J.M., Klimkiewicz A., Wnorowska A., Brower K.J., Wojnar M. Relationship between emotional processing, drinking severity and relapse in adults treated for alcohol dependence in Poland. Alcohol. Alcohol. 2015;50(2):173–179. doi: 10.1093/alcalc/agu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopera M., Trucco E.M., Jakubczyk A., Suszek H., Kobylinski P., Wojnar M., Zucker R.A. Relationship between Alcohol-related family adversity, alcohol use across adolescence, and mental states recognition in young adulthood. J. Addict. Med. 2020;14(5):e247–e256. doi: 10.1097/ADM.0000000000000659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koven N.S., Thomas W. Mapping facets of alexithymia to executive dysfunction in daily life. Personal. Individ. Differ. 2010;49(1):24–28. doi: 10.1016/j.paid.2010.02.034. [DOI] [Google Scholar]
- Laloyaux J., Fantini C., Lemaire M., Luminet O., Laroi F. Evidence of contrasting patterns for suppression and reappraisal emotion regulation strategies in alexithymia. J. Nerv. Ment. Dis. 2015;203(9):709–717. doi: 10.1097/NMD.0000000000000353. [DOI] [PubMed] [Google Scholar]
- Lanza S.T., Russell M.A., Braymiller J.L. Emergence of electronic cigarette use in US adolescents and the link to traditional cigarette use. Addict. Behav. 2017;67:38–43. doi: 10.1016/j.addbeh.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C., Gu H., Loughead J., Ruparel K., Yang Y., Stein E.A. Large-Scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry. 2014;71(5):523. doi: 10.1001/jamapsychiatry.2013.4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Mai X., Liu C. The default mode network and social understanding of others: what do brain connectivity studies tell us. Front. Hum. Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston J.A., Chen C., Kwon M., Park E. Physical and mental health outcomes associated with adolescent E-cigarette use. J. Pediatr. Nurs. 2022;64:1–17. doi: 10.1016/j.pedn.2022.01.006. (Get rights and content) [DOI] [PubMed] [Google Scholar]
- Lorenzetti V., Gaillard A., Beyer E., Kowalczyk M., Kamboj S.K., Manning V., Gleeson J. Do mindfulness-based interventions change brain function in people with substance dependence? A systematic review of the fMRI evidence. BMC Psychiatry. 2023;23(1) doi: 10.1186/s12888-023-04789-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loughead J., Ray R., Wileyto E.P., Ruparel K., Sanborn P., Siegel S., Gur R.C., Lerman C. Effects of the α4β2 partial agonist varenicline on brain activity and working memory in abstinent smokers. Biol. Psychiatry. 2010;67(8):715–721. doi: 10.1016/j.biopsych.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Loughead J., Wileyto E.P., Ruparel K., Falcone M., Hopson R., Gur R., Lerman C. Working Memory-Related neural activity predicts future smoking relapse. Neuropsychopharmacology. 2015;40(6):1311–1320. doi: 10.1038/npp.2014.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luminet O., Nielson K.A., Ridout N. Having no words for feelings: alexithymia as a fundamental personality dimension at the interface of cognition and emotion. Cogn. Emot. 2021;35(3):435–448. doi: 10.1080/02699931.2021.1916442. [DOI] [PubMed] [Google Scholar]
- Miech R.A., Johnston L.D., Patrick M.E., O'Malley P.M., Bachman J.G. Monitoring the Future Monograph Series. Institute for Social Research, University of Michigan; Ann Arbor, MI: 2024. Monitoring the future national survey results on drug use, 1975–2023: overview and detailed results for secondary school students. [Google Scholar]
- Miech R.A., Johnston L.D., Patrick M.E., O'Malley P.M. Monitoring the Future Series. Institute for Social Research, The University of Michigan; Ann Arbor MI: 2024. Monitoring the future national survey results on drug use 1975-2023: overview and detailed results for secondary school students. [Google Scholar]
- Moriguchi Y., Decety J., Ohnishi T., Maeda M., Mori T., Nemoto K., Matsuda H., Komaki G. Empathy and judging other's pain: an fMRI study of alexithymia. Cereb. Cortex. 2007;17(9):2223–2234. doi: 10.1093/cercor/bhl130. [DOI] [PubMed] [Google Scholar]
- Morra S., Parrella I., Camba R. The role of working memory in the development of emotion comprehension. Br. J. Dev. Psychol. 2011;29(4):744–764. doi: 10.1348/2044-835x.002006. [DOI] [PubMed] [Google Scholar]
- Moskal K., Teeters J., McCollum D. Examining differences in emotion dysregulation between emerging adult Alcohol-Only users, abstainers, and simultaneous users. Cannabis. 2023 doi: 10.26828/cannabis/2023/000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau S.E. Chapter 15 - neural mechanisms of emotions, alexithymia, and depression. Handb. Clin. Neurol. 2021;183:299–313. doi: 10.1016/B978-0-12-822290-4.00014-1. [DOI] [PubMed] [Google Scholar]
- Nejati V., Majdi R., Salehinejad M.A., Nitsche M.A. The role of dorsolateral and ventromedial prefrontal cortex in the processing of emotional dimensions. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-81454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols T.E., Das S., Eickhoff S.B., Evans A.C., Glatard T., Hanke M., Kriegeskorte N., Milham M.P., Poldrack R.A., Poline J.-B., Proal E., Thirion B., Van Essen D.C., White T., Yeo B.T.T. Best practices in data analysis and sharing in neuroimaging using MRI. Nat. Neurosci. 2017;20(3):299–303. doi: 10.1038/nn.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen A.M., McMillan K.M., Laird A.R., Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwardhan I., Mason W.A., Chmelka M.B., Savolainen J., Miettunen J., Järvelin M.-R. Prospective relations between alexithymia, substance use and depression: findings from a national birth cohort. Nord. J. Psychiatry. 2019;73(6):340–348. doi: 10.1080/08039488.2019.1634758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit G., Luminet O., Maurage F., Tecco J., Lechantre S., Ferauge M., Gross J.J., De Timary P. Emotion regulation in alcohol dependence. Alcohol. Clin. Exp. Res. 2015;39(12):2471–2479. doi: 10.1111/acer.12914. [DOI] [PubMed] [Google Scholar]
- Preacher K.J., Hayes A.F. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav. Res. Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Qin P., Northoff G. How is our self related to midline regions and the default-mode network? NeuroImage. 2011;57(3):1221–1233. doi: 10.1016/j.neuroimage.2011.05.028. [DOI] [PubMed] [Google Scholar]
- R Studio Team. (2020). RStudio: Integrated Development for R. In Retrieved from 〈http://www.rstudio.com/〉.
- Reesor L., Vaughan E.M., Hernandez D.C., Johnston C.A. Addressing outcomes expectancies in behavior change. Am. J. Lifestyle Med. 2017;11(6):430–432. doi: 10.1177/1559827617722504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reker M., Ohrmann P., Rauch A.V., Kugel H., Bauer J., Dannlowski U., Arolt V., Heindel W., T S. Individual differences in alexithymia and brain response to masked emotion faces. Cortex. 2010;46(5):658–667. doi: 10.1016/j.cortex.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Riadh O., Naoufel O., Rejeb M.R.B., Gall D.L. Neuro-cognitive correlates of alexithymia in patients with circumscribed prefrontal cortex damage. Neuropsychologia. 2019;135 doi: 10.1016/j.neuropsychologia.2019.107228. [DOI] [PubMed] [Google Scholar]
- Rocca M.A., Valsasina P., Hulst H.E., Abdel-Aziz K., Enzinger C., Gallo A., Pareto D., Riccitelli G., Muhlert N., Ciccarelli O., Barkhof F., Fazekas F., Tedeschi G., Arévalo M.J., Filippi M. Functional correlates of cognitive dysfunction in multiple sclerosis: a multicenter fMRI study. Hum. Brain Mapp. 2014;35(12):5799–5814. doi: 10.1002/hbm.22586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M.D., Martinez S.A., Rapuano K.M., Conley M.I., Cohen A.O., Cornejo M.D., Hagler D.J., Meredith W.J., Anderson K.M., Wager T.D., Feczko E., Earl E., Fair D.A., Barch D.M., Watts R., Casey B.J. Behavioral and neural signatures of working memory in childhood. J. Neurosci. 2020;40(26):5090–5104. doi: 10.1523/jneurosci.2841-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseel Y. lavaan R. Package Struct. Equ. Model. 2012 〈https://www.jstatsoft.org/v48/i02/〉 Retrieved 2 from. [Google Scholar]
- Sajonz B., Kahnt T., Margulies D.S., Park S.Q., Wittmann A., Stoy M., Ströhle A., Heinz A., Northoff G., Bermpohl F. Delineating self-referential processing from episodic memory retrieval: common and dissociable networks. NeuroImage. 2010;50(4):1606–1617. doi: 10.1016/j.neuroimage.2010.01.087. [DOI] [PubMed] [Google Scholar]
- Salles B.M., Maturana De Souza W., Dos Santos V.A., Mograbi D.C. Effects of DBT-based interventions on alexithymia: a systematic review. Cogn. Behav. Ther. 2023;52(2):110–131. doi: 10.1080/16506073.2022.2117734. [DOI] [PubMed] [Google Scholar]
- Samimi z, Kord M., Mirdoraghi F., Hasani J. The effectiveness of emotional Stimulus-based working memory training in improving the cognitive emotion regulation in adolescents with Post- traumatic stress disorder. J. Kerman Univ. Med. Sci. 2017;24(4):298–311. 〈https://jkmu.kmu.ac.ir/article_53932_7aa77bc0732f4206b1f9bdf65a55dcbe.pdf〉 [Google Scholar]
- Satterthwaite T.D., Ciric R., Roalf D.R., Davatzikos C., Bassett D.S., Wolf D.H. Motion artifact in studies of functional connectivity: characteristics and mitigation strategies. Hum. Brain Mapp. 2019;40(7):2033–2051. doi: 10.1002/hbm.23665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman E.P., Smith A.R., Silva K., Icenogle G., Duell N., Chein J., Steinberg L. The dual systems model: review, reappraisal, and reaffirmation. Dev. Cogn. Neurosci. 2016;17:103–117. doi: 10.1016/j.dcn.2015.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sifneos P.E. The prevalence of 'alexithymic' characteristics in psychosomatic patients. Psychother. Psychosom. 1973;22:255–262. doi: 10.1159/000286529. 2/6. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Dev. Rev. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland B.D., Fallah-Sohy N., Kopera M., Jakubczyk A., Sutherland M.T., Trucco E.M. Alexithymia mediates the association between childhood trauma and adolescent E-cigarette use. Drug Alcohol Depend. 2022;236(1) doi: 10.1016/j.drugalcdep.2022.109500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland B.D., Sutherland M.T., Trucco E.M. Electronic cigarette use intentions mediate the association between low Self-Control and future use by internalizing symptoms. Subst. Use amp Misuse. 2022;57(12):1797–1807. doi: 10.1080/10826084.2022.2115848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland B.D., Viera Perez P.M., Crooks K.E., Flannery J.S., Hill-Bowen L.D., Riedel M.C., Laird A.R., Trucco E.M., Sutherland M.T. The association of amygdala-insula functional connectivity and adolescent e-cigarette use via sleep problems and depressive symptoms. Addict. Behav. 2022;135 doi: 10.1016/j.addbeh.2022.107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland M.T., Stein E.A. Functional neurocircuits and neuroimaging biomarkers of tobacco use disorder. Trends Mol. Med. 2018;24(2):129–143. doi: 10.1016/j.molmed.2017.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland M.T., McHugh M.J., Pariyadath V., Stein E.A. Resting state functional connectivity in addiction: lessons learned and a road ahead. NeuroImage. 2012;62(4):2281–2295. doi: 10.1016/j.neuroimage.2012.01.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland M.T., Ray K.L., Riedel M.C., Yanes J.A., Stein E.A., Laird A.R. Neurobiological impact of nicotinic acetylcholine receptor agonists: an activation likelihood estimation Meta-Analysis of pharmacologic neuroimaging studies. Biol. Psychiatry. 2015;78(10):711–720. doi: 10.1016/j.biopsych.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland M.T., Riedel M.C., Flannery J.S., Yanes J.A., Fox P.T., Stein E.A., Laird A.R. Chronic cigarette smoking is linked with structural alterations in brain regions showing acute nicotinic drug-induced functional modulations. Behav. Brain Funct. 2016;12(1) doi: 10.1186/s12993-016-0100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarter R.E., Kirisci L., Mezzich A., Cornelius J.R., Pajer K., Vanyukov M., Gardner W., Blackson T., Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am. J. Psychiatry. 2003;160(6):1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Taylor G.J., Bagby R.M. New trends in alexithymia research. Psychother. Psychosom. 2004;73(2):68–77. doi: 10.1159/000075537. [DOI] [PubMed] [Google Scholar]
- Thompson T.W., Waskom M.L., Gabrieli J.D.E. Intensive working memory training produces functional changes in Large-scale frontoparietal networks. J. Cogn. Neurosci. 2016;28(4):575–588. doi: 10.1162/jocn_a_00916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasino B., Fabbro F. Increases in the right dorsolateral prefrontal cortex and decreases the rostral prefrontal cortex activation after-8 weeks of focused attention based mindfulness meditation. Brain Cogn. 2016;102:46–54. doi: 10.1016/j.bandc.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Trucco E.M., Cristello J.V., Sutherland M.T. Do parents still matter? The impact of parents and peers on adolescent electronic cigarette use. J. Adolesc. Health. 2021;68(4):780–786. doi: 10.1016/j.jadohealth.2020.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine G., Sofuoglu M. Cognitive effects of nicotine: recent progress. Curr. Neuropharmacol. 2018;16(4):403–414. doi: 10.2174/1570159x15666171103152136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen N., Domachowska I., Nileson K.A. In: Alexithymia: Advances in Research, Theory, and Clinical Practice. Luminet In.O., Bagby R.M., Taylor G.J., editors. Cambridge University Press; 2018. Memory and executive functions in alexithymia. [Google Scholar]
- Vik P.W., Cellucci T., Jarchow A., Hedt J. Cognitive impairment in substance abuse. Psychiatr. Clin. North Am. 2004;27(1):97–109. doi: 10.1016/s0193-953x(03)00110-2. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Blanco C. Substance use disorders: a comprehensive update of classification, epidemiology, neurobiology, clinical aspects, treatment and prevention. World Psychiatry. 2023;22(2):203–229. doi: 10.1002/wps.21073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Pan D., Li X. In. Springer International Publishing; 2019. The neural mechanism of working memory training improving emotion regulation; pp. 72–81. [DOI] [Google Scholar]
- Weissman D.H., Roberts K.C., Visscher K.M., Woldorff M.G. The neural bases of momentary lapses in attention. Nat. Neurosci. 2006;9(7):971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wilcox C.E., Pommy J.M., Adinoff B. Neural circuitry of impaired emotion regulation in substance use disorders. Am. J. Psychiatry. 2016;173(4):344–361. doi: 10.1176/appi.ajp.2015.15060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiu L., Zhou R., Jiang Y. Working memory training improves emotion regulation ability: evidence from HRV. Physiol. Behav. 2016;155(1):25–29. doi: 10.1016/j.physbeh.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Yıldırım Ayaz E., Dincer B. THE LEVEL OF RUMINATIVE THOUGHT AND ALEXITHYMIA OF PEOPLE IN THE COVID-19 PANDEMIC. Process. Psychiatr. Danub. 2021;33(2):240–247. doi: 10.24869/psyd.2021.240. [DOI] [PubMed] [Google Scholar]
- Zevin J. Encyclopedia of Neuroscience. Elsevier Ltd; 2009. Word recognition. [Google Scholar]
- Zhang L., Zhu C., Ye R., Cao Z., Tian Y., Yang P., Hu P., K W. Impairment of conflict processing in alexithymic individuals. Neurosci. Lett. 2011;504(3):261–264. doi: 10.1016/j.neulet.2011.09.043. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Fu J., Zhao X. Neural correlates of working memory training: an fMRI meta-analysis. NeuroImage. 2024;301(1) doi: 10.1016/j.neuroimage.2024.120885. [DOI] [PubMed] [Google Scholar]
- Zhou H., Chen X., Shen Y., Li L., Chen N., Zhu Z., Castellanos F.X., Yan C. Rumination and the default mode network: Meta-analysis of brain imaging studies and implications for depression. NeuroImage. 2020;206(1) doi: 10.1016/j.neuroimage.2019.116287. [DOI] [PubMed] [Google Scholar]
- Zimmermann K., Walz C., Derckx R.T., Kendrick K.M., Weber B., Dore B., Ochsner K.N., Hurlemann R., Becker B. Emotion regulation deficits in regular marijuana users. Hum. Brain Mapp. 2017;38(8):4270–4279. doi: 10.1002/hbm.23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
Data will be made available on request.