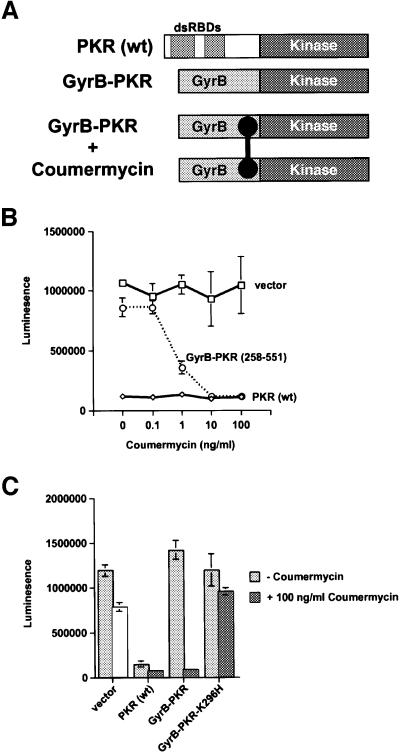
Fig. 6. Coumermycin-induced activation of GyrB–PKR fusion proteins in mammalian cells. (A) Schematics of wild-type PKR [PKR(wt)]; GyrB–PKR fusion protein consisting of the N-terminal 220 amino acids of E.coli GyrB fused to the human PKR kinase domain residues 258–551 (GyrB–PKR); and coumermycin (black dumbbell)-mediated dimerization of GyrB–PKR fusion proteins (GyrB–PKR + coumermycin). (B) NIH 3T3 cells were co-transfected with the luciferase reporter plasmid pGL3-Control (Promega) and either empty vector (vector, pC869), or plasmids to express either wild-type PKR [PKR (wt), pC882] or the GyrB–PKR fusion protein [GyrB–PKR (258–551), pC939], as indicated. Twenty-four hours following transfection, cells were treated with dimethylsulfoxide (DMSO) alone or the indicated concentration of coumermycin dissolved in DMSO. After another 24 h, cells were harvested, lysed and samples of the whole-cell extracts were assayed for luciferase activity. The results are the average and standard deviation from three independent experiments. (C) NIH 3T3 cells were co-transfected with the luciferase reporter plasmid pGL3-Control (Promega) and either empty vector (vector, pC869), or plasmids to express wild-type PKR [PKR (wt), pC882], GyrB–PKR (pC939), or GyrB–PKR-K296H (pC940), as indicated. Twenty-four hours following transfection, cells were treated with DMSO alone (–coumermycin) or with 100 ng/ml coumermycin dissolved in DMSO. Following 24 h stimulation, cells were harvested, lysed and samples of the whole-cell extracts were assayed for luciferase activity. The results are the average and standard deviation from three independent experiments.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.