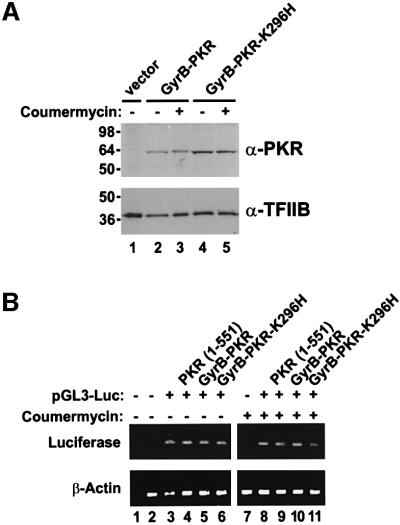
Fig. 7. Coumermycin treatment does not alter GyrB–PKR expression or luciferase reporter mRNA levels in NIH 3T3 cell transfectants. (A) Analysis of PKR expression. NIH 3T3 cells were co-transfected with the luciferase reporter plasmid pGL3-Control (Promega) and either empty vector (lane 1, vector, pC869), or plasmids to express GyrB–PKR (lanes 2 and 3, pC939) or GyrB–PKR-K296H (lanes 4 and 5, pC940), as indicated. Twenty-four hours following transfection, cells were treated with DMSO alone (– coumermycin) or with 100 ng/ml coumermycin dissolved in DMSO (+ coumermycin). Following 24 h stimulation with the drug, cells were harvested, lysed and samples of the whole-cell extracts were subjected to SDS–PAGE and then immunoblotted with anti-PKR (upper panel) or anti-TFIIB (lower panel) antisera as indicated. (B) Analysis of luciferase mRNA levels. NIH 3T3 cells were co-transfected with the luciferase reporter plasmid pGL3-Control (pGL3-Luc) or empty vector (pC869), as indicated, and either empty vector (no label, pC869) or plasmids to express wild-type PKR [PKR (1–551), pC882], GyrB–PKR (pC939), or GyrB–PKR-K296H (pC940), as indicated. Twenty-four hours following transfection, cells were treated with DMSO alone (– coumermycin) or with 100 ng/ml coumermycin dissolved in DMSO (+ coumermycin). Following 24 h stimulation, cells were harvested, lysed and the amount of luciferase reporter and β-actin mRNAs was determined by RT–PCR, as described previously (Kawagishi-Kobayashi et al., 2000). Lanes 1 and 2 are control experiments for the RT–PCR analysis in which either cellular RNA was omitted (lane 1) or the RNA was obtained from non-transfected cells (lane 2).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.