Abstract
Background
Diabetic retinopathy (DR) is a microvascular complication of diabetes characterized by damage to the retina’s neurons and blood vessels. Ginkgo biloba extract (GBE) has demonstrated neuroprotective properties, however, its specific mechanisms in DR remain incompletely understood. This research aims to elucidate the underlying mechanisms of GBE in DR.
Methods and results
A diabetic rat model was induced with streptozotocin (STZ) and divided into control, diabetic, and GBE-treated groups. Retinal tissues of each group were analyzed using histology, TUNEL staining, and immunofluorescence. Bioinformatics identified potential GBE targets for DR, and protein-protein interaction network analysis prioritized core targets. Western blot and immunoprecipitation assays were used to detect protein expression and ubiquitination status. We successfully constructed the DR rat models and observed that GBE intervention effectively reverses diabetes-induced hyperglycemia and mitigates retinal ganglion cell (RGC) damage in the DR rat model. TUNEL staining indicates GBE’s protective role against RGC apoptosis induced by DR. Bioinformatics identified 135 GBE targets in DR, with a focus on apoptosis pathways. Critically, we demonstrated an upregulation of TP53 expression in the retinal tissues of the DR rat model, an effect that was successfully reversed following GBE intervention. Notably, GEB increased TP53 ubiquitination, suggesting a potential modulation of TP53 stability and function.
Conclusions
GBE attenuates DR progression by modulating TP53 ubiquitination in a rat model. The findings highlight the potential therapeutic benefits of GBE in DR and suggest further investigation into its mechanisms and broader bioactivity pathways.
Keywords: Ginkgo biloba extract, Diabetic retinopathy, Retinal ganglion cell, TP53, Ubiquitination
Introduction
Vision loss from optic neuropathies, primarily due to the degeneration of retinal ganglion cells (RGCs) in the ganglion cell layer (GCL), poses a significant health challenge [1, 2]. RGCs are crucial neurons in the retina, transmitting visual signals to the brain via the optic nerve. Their axons form the optic nerve fibers, and the preservation and functionality of these fibers are crucial for maintaining normal vision [3]. Conditions like glaucoma and ischemic optic neuropathy cause RGC apoptosis and axon damage, leading to visual field defects and, in extreme cases, blindness [4–7]. These degenerative processes involve complex molecular mechanisms, including mitochondrial dysfunction, neurotrophin deprivation, oxidative stress, and neuroinflammation [8, 9].
Diabetes mellitus, a chronic metabolic disorder characterized by persistent hyperglycemia, poses a multifaceted threat to various bodily organs and systems, including the eyes [10, 11]. This condition affects glucose metabolism, triggering a cascade of pathophysiological processes that can lead to severe ocular complications. It is noteworthy that in the pathological progression of Diabetic Retinopathy (DR), damage to RGCs occurs much earlier than the microvascular lesions traditionally recognized [12]. In early diabetes, RGCs may exhibit structural and functional abnormalities, including changes in cell structure, metabolic issues, and disruptions in signal conduction [13, 14]. These often result from hyperglycemia-induced oxidative stress, inflammation, and vascular damage [15–17]. Hyperglycemia leads to retinal vessel thickening, narrowed lumens, reduced blood flow, and ischemia/hypoxia, exacerbating RGC damage. As diabetes progresses, RGC degeneration intensifies, leading to vision decline, defects, and blindness [18]. Hence, understanding the mechanisms of RGC degeneration is crucial for developing effective treatments.
DR treatments focus on regulating vascular changes, inflammation, and oxidative stress with antidiabetic drugs to lessen the impact of hyperglycemia. Currently, dietary supplements have shown the potential to ease DR symptoms [19]. Among dietary supplements, flavonoids have garnered considerable attention due to their association with a decreased risk of progressive retinal degeneration. As a subclass of plant-derived polyphenols, they promote health by exerting antioxidant properties and anti-inflammatory effects and modulating cellular damage signaling pathways [20, 21]. Ginkgo biloba extract (GBE), which contains various flavone glycosides and terpenoids [22], holds significant promise in medicinal applications. GBE is renowned for its robust antioxidant capabilities, serving as a free radical scavenger that efficiently counteracts oxidative stress by neutralizing reactive oxygen and nitrogen species [23, 24]. Moreover, GBE has demonstrated the ability to suppress the increase in oxidative decomposition products of low-density lipoprotein (LDL), a pivotal step in the pathogenesis of atherosclerosis and cardiovascular diseases [25]. Additionally, it is one of the most extensively studied herbal remedies for cognitive disorders and Alzheimer’s disease [26, 27]. Currently, the neuroprotective potential of GBE in retinal diseases is gradually unraveling the mystery [21, 28]. Nevertheless, its effectiveness in preventing and treating DR remains controversial. While some studies highlight promising results, showing significant improvements in visual acuity and retinal health, others report limited efficacy and inconsistent outcomes, necessitating further research to establish definitive guidelines for its clinical application.
Materials and methods
Establishment and administration of the DR model in rats
The animal experiments were conducted according to procedures following the Guiding Principles in the Care and Use of Animals (China). We constructed the streptozotocin (STZ)-induced diabetes model, which are well-established and shared across both type 1 and type 2 diabetes [29–33], to elucidate early pathophysiological mechanisms of diabetic retinopathy. Twenty-five male Sprague-Dawley (SD) rats (body weight: 180–200 g) were purchased from Slaccas (Shanghai, China) and acclimatized for 1 week under standardized laboratory conditions (temperature: 22 ± 2 °C; Humidity: 55 ± 5%; 12 h light/dark cycle) with standard chow and water. The rats were then divided into two groups: a model group (n = 18) and a control group (n = 7). Diabetes was induced in the model group through intraperitoneal injection of STZ (Yuanye Bio-Technology Co., Ltd., Shanghai, China) at a dose of 60 mg/kg body weight, dissolved in 0.1 M citrate buffer (pH = 4.5, 1% concentration). The control group received an equivalent volume of citrate buffer without STZ. Fasting blood glucose (FBG) levels were monitored from the tail vein every 3 days starting 1 weeks post-injection using a glucometer (590, Yuyue Medical Equipment and Supply Co., Ltd., Jiangsu, China). Rats with FBG ≥ 17 mmol/L for ≥ 80% of the cohort were considered to have successfully induced diabetes, with control rats maintaining normoglycemia (FBG < 5.6 mmol/L). 8 weeks after STZ injection, the diabetic model rats were randomly divided into two groups: a non-treated diabetic group (model group; n = 10) and a GBE-treated group (GBE group; n = 8). The GBE group received daily oral gavage of GBE (CAS No.: 90045-36-6; Art.No.: XW900453661, SINOPHARM, Shanghai, China; containing 24% flavonoids and 6% terpenes, https://www.chemicalbook.com/CAS_90045-36-6.htm) at a dose of 150 mg/kg/day for 30 days. Based on the treatment duration of the model involving prior medications [34, 35], after the treatment regimen was completed in this study, the remaining rats were euthanized for terminal analysis after their FBG levels were measured on empty stomachs. The eyeballs of the rats were enucleated. One eye from each rat was fixed in an ocular fixation solution (G1109, Servicebio®, Wuhan, China), and the anterior segment was removed. The posterior segment, including the retina, was then embedded in paraffin for histological analysis. The contralateral eye from each rat was isolated and stored at −80 °C for subsequent experiments.
Hematoxylin and Eosin (H&E) staining for histological assessment
At post-modeling, a preliminary histological evaluation was conducted by euthanizing one control rat and two diabetic rats (one from each subgroup: model group and GBE-treated group). The eyeballs were enucleated, and the retinas were separated and fixed in an ocular fixation solution for the H&E staining. Briefly, Fixed tissues were dehydrated in a graded ethanol series (70%, 80%, 95%, and 100%) for 5 min each, cleared in xylene (10023418, SINOPHARM) for 20 min, and then embedded in paraffin wax. Slices (5 μm thickness) were cut using a microtome (RM2016, Leica, Germany), incubated at 60 °C for 30 min, deparaffinized in xylene for 10 min, and rehydrated through a descending ethanol series (100%, 95%, 80%, and 70%). Then, slices were stained with a High-definition constant staining kit for H&E (G1076, Servicebio®) following the manufacturer’s specifications. Slices were mounted with neutral balsam (10004160, SINOPHARM), and visualized under a light microscope (E100, Nikon, Japan) equipped with an imaging system (DS-U3, Nikon). Images were captured at 200× and 400× magnification. Upon observation of significant retinal lesions, all remaining rats were euthanized, and their eyeballs were fixed for histological examination after paraffin embedding.
Immunofluorescence (IF)
The retinal tissue slices were sequentially immersed in eco-friendly dewaxing solutions (G1128, Servicebio®) for 10 min, followed by anhydrous ethanol for 5 min. Then, the tissue slices were permeabilized with a 20× citric acid antigen retrieval solution (G1202, Servicebio®) in phosphate-buffered saline (PBS) for 15 min. After slightly drying, slices were outlined with a histochemical pen and incubated with bovine serum albumin (BSA, GC305010, Servicebio®) for 15 min to block. The primary antibody against the Gene - RNA Binding Protein, MRNA Processing Factor (RBPMS) (15187-1-AP, proteintech®, Wuhan, China) was applied to the tissue sections and incubated overnight at 4 °C, followed by incubating the diluted Donkey anti-Rabbit IgG (H + L) (A21207, Invitrogen, USA), a fluorescent secondary antibody, for 1 h at room temperature. The slides were incubated in PBS (pH 7.4) with DAPI staining reagent (G1012, Servicebio®) for 10 min in the dark. Finally, Slices were coverslipped with antifade mounting medium (G1401, Servicebio®) and imaged using a laser confocal scanning microscope (LSM880, Zeiss, Germany).
TdT-mediated dUTP Nick-End labeling (TUNEL) staining
The TUNEL assay was performed to detect cell apoptosis in the designated retinal tissues. Following paraffin embedding, the tissues were sectioned and de-paraffinized. Subsequent procedures followed the manufacturer’s instructions for the TUNEL cell apoptosis detection kit (ab66110, Abcam, Cambridge, UK). Finally, the results were observed under a microscope.
Screening and acquisition of potential targets associated with GBE and DR
To identify potential targets for DR, a comprehensive search was conducted. Using “Ginkgo Biloba Extract” or “Ginkgo Folium” (the different expression forms of GBE) as keywords, we screened compounds in the TCMSP (https://www.tcmsp-e.com) and SYMMAP (http://www.symmap.org/) databases based on oral bioavailability (OB) > 30% and drug-likeness (DL) > 0.18 as unique targets associated with GBE. Using the keyword “Diabetic retinopathy” across three reputable databases: Genecards (https://www.genecards.org/), OMIM (https://www.omim.org/), and DisGeNET (https://disgenet.com/), the targets retrieved from these searches were considered as candidate targets associated with DR. By intersecting the target points related to GBE with the potential target points for DR, we obtain the potential target points for treating DR with GBE, presented with a Venn diagram.
Bioinformatics analysis
The identified potential targets were input into the STRING database (https://cn.string-db.org/) to obtain the potential target protein-protein interaction (PPI) network diagram, selecting high confidence levels (> 0.7), with all other settings set to default values. Then, the PPI network was imported into Cytoscape 3.7.2 software for topological analysis. The topological analysis provides three key metrics: “Degree”, “Closeness”, and “Betweenness”. These are commonly used to measure the topological position of a node within a PPI network, where “Degree” and “Closeness” reflect the node’s importance and criticality, and “Betweenness” indicates the node’s proximity to other nodes. Targets with all three metrics above the median are selected as core targets for the GBE treatment of DR. Using the R software package ‘’clusterProfiler’’ to perform Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis on potential targets of GBE treatment for DR.
Western blotting (WB)
Rat tissue samples were collected, and protein extracts were prepared with RIPA lysis buffer on ice. The total protein concentration was measured with the BCA protein assay kit (PC0020, Solarbio, Beijing, China). Then, 20 µg of each protein sample was separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride (PVDF) membrane (88585, Thermo Fisher, USA). After blocking nonspecific binding and incubating with primary and secondary antibodies, the protein bands were visualized using an ECL kit (BL520B, Biosharp, Hefei, China). Table 1 lists the antibodies used.
Table 1.
Details of antibodies
| Name | Brand | Number |
|---|---|---|
| MDM2 | proteintech® | 66511-1-Ig |
| MYC | CST | #5605 |
| TP53 | CST | #2524 |
| BCL-2 | proteintech® | 26593-1-AP |
| β-actin | proteintech® | 20536-1-AP |
| Anti-rabbit IgG, HRP-linked Antibody | CST | 7074 S |
| Anti-mouse IgG, HRP-linked Antibody | Beyotime | A0216 |
Real-Time quantitative polymerase chain reaction (RT-qPCR)
We used Trizol reagent (15596018, Ambion, China) to extract total RNA. After that, chloroform was used for separation, and isopropanol (327270010, Acros Organics, USA) was employed for precipitation. The precipitated RNA underwent cleaning with 75% ethanol, was air-dried, dissolved in RNase-free water, and then quantified. For cDNA synthesis, the PrimeScript™ RT reagent Kit (RR037Q, TAKARA, Japan) was applied. RT-qPCR analysis was carried out using the TB Green™ Premix Ex Taq™ II kit (RR820S, TAKARA, Japan), with β-actin serving as the internal reference gene. The relative gene expression levels were calculated using the 2−ΔΔCT method. The specific primer sequences used are shown in Table 2.
Table 2.
Details of primer sequences
| Primer name | Primer sequence |
|---|---|
| TP53 |
F: GCCATCTACAAGAAGTCACA R: TCGTCCAGATACTCAGCATA |
| MYC |
F: CAGATCCCTGAGTTGGAAAA R: TTATGCACCAGAGTTTCGAA |
| BCL2 |
F: GTGGATGACTGAGTACCTGA R: AGCCAGGAGAAATCAAACAG |
| CASP3 |
F: ATTGAGACAGACAGTGGAAC R: AGTGAGGATGTGCATGAATT |
| CASP9 |
F: TAAGAAAATGGTCACGGCTT R: TCACAATTCTCTCGATGGAC |
| MDM2 |
F: AAGTGACCATTCTGCTGATT R: CTTTCTCCTGCCTGATAGAC |
| SERPINE1 |
F: CAATGGAAGAGCAACATGAC R: TGACCTTTTGTAGTGCTTGT |
| EGFR |
F: CTCCAGAGGATGTTCAACAA R: ATTTCCTGTAAGTTCCGCAT |
| TLR4 |
F: TGTGAGCATTGATGAGTTCA R: GGTAGACTCAGCTTTGGAAA |
| PTGS2 |
F: CTCCAACCTCTCCTACTACA R: TGCGAACATCATATTTGTGC |
| PPARG |
F: AGTTCAAACATATCACCCCC R: CAGAGTCACTTGGTCATTCA |
| β-actin |
F: AACACAGTGCTGTCTGGTG R: GTAACAGTCCGCCTAGAAGC |
Immunoprecipitation (IP)
Retinal tissues from DR model rats (untreated and GBE-treated groups) and control rats were homogenized in ice-cold IP lysis buffer (P0013, Beyotime, Shanghai, China); supplemented with protease inhibitor cocktail (P8340, Merck Millipore, USA). Lysates were centrifuged at 12,000× g for 15 min at 4 °C. Supernatants were collected, and protein concentrations were quantified using a BCA assay kit. The input group consisted of 20 µg of protein lysate mixed with an equal 2× SDS buffer, boiled for 10 min, and then subjected to a WB assay with TP53-specific primary antibody (2524, Cell Signaling Technology, USA). Lysates, each containing 500 µg of total protein, were incubated overnight at 4 °C with a mouse anti-TP53 monoclonal antibody. Immune complexes were captured by adding Flag beads (M8823, Merck Millipore) and incubating for an additional 2 h at 4 °C. Following incubation and washing, the remaining Liquid was removed, and 20 µL of 1× SDS buffer was added. The mixture was then boiled for 10 min to denature the proteins. The immunoprecipitated proteins were then probed with an anti-ubiquitin (UB) primary antibody (3936, Cell Signaling Technology) for WB assays to detect ubiquitination modifications.
Statistical analysis
Statistical analysis was conducted using GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA). Two-tailed unpaired Student’s t-tests were performed to compare differences between the two groups. Significance was set at p < 0.05. All quantitative data were based on three or more replicates.
Results
GBE intervention mitigates diabetes-induced RGC damage in a diabetic rat model
To elucidate the effect of GBE on DR, we Successfully established a diabetic rat model using 25 male SD rats. The model group (n = 18) received STZ (60 mg/kg) in citrate buffer (pH 4.5, 1%) to induce diabetes, while the control group (n = 7) received buffer only (The schematic timeline of the experiment as shown in Fig. 1A). FBG levels were monitored every 3 days beginning 1 weeks after STZ injection to confirm hyperglycemia. By the end of this monitoring period, over 80% of the STZ-treated rats exhibited FBG levels exceeding 17 mmol/L. Whereas control rats maintained normoglycemia (FBG < 5.6 mmol/L), confirming the successful induction of diabetes (Fig. 1B). Eight weeks after STZ injection, hyperglycemic rats were divided into a non-treated group (n = 10) and a GBE-treated group (n = 8) that received daily oral gavage of GBE (150 mg/kg) for 30 days. No adverse physiological or behavioural effects were observed following GBE administration in any of the treated animals throughout the study period. At the experimental endpoint (about 12 weeks post-STZ injection), all remaining rats were euthanized. Preliminary histological assessment of retinas (from one control and two diabetic rats euthanized) revealed structural disorganization and GCL thinning in diabetic animals, suggesting progressive retinal pathology (results not shown). Subsequently, full analysis was conducted on all terminal samples. Our data indicated that although GBE intervention successfully reversed the model group’s stimulatory effect on FBG levels (Fig. 1C), FBG levels were still significantly elevated after treatment (~ 20 mmol/L). H&E staining of the retinal tissues showed significant degeneration of RGCs in the diabetic model group compared to the controls, characterized by reduced cell density, disordered cell structure, and cytoplasmic vacuolization. In contrast, GBE-treated diabetic model rats exhibited partial preservation of RGC morphology, with attenuated cellular shrinkage and improved structural integrity in the GCL (Fig. 1D). For further investigation, we conducted IF staining to detect the levels of RBPMS, a well-established biomarker for RGCs [36]. The diabetic model group showed reduced RBPMS expression, suggesting RGC loss or dysfunction. GBE-treated rats, however, partially restored RBPMS levels, suggesting protective effects against diabetes-induced RGC damage (Fig. 2A). TUNEL staining revealed few apoptotic cells in the control group, but a significant increase in apoptotic RGCs in the diabetic model. Importantly, GBE-treated rats showed fewer TUNEL-positive cells, indicating a protective role against diabetes-induced RGC apoptosis (Fig. 2B). These outcomes suggest that GBE intervention mitigates diabetes-induced damage to RGC, highlighting its potential therapeutic benefits in DR.
Fig. 1.
GBE intervention mitigates diabetes-induced RGC damage in a diabetic rat model. (A) Schematic timeline of the experimental design to visually illustrate the sequence and duration of diabetes induction and GBE treatment. (B) FBG levels detection in control and diabetic model rats at different time points. (C) FBG levels detection in the control, model, and GBE-treated groups after 30 days of administration treatment. (D) Retinal tissues isolated from control, model, and GBE-treated rats were processed into paraffin sections, and the degeneration of RGCs in the GCL was assessed by H&E staining. Scale bars represent 100 μm (200×) and 50 μm (400×). All experiments included appropriate controls and were repeated at least three times to ensure reproducibility. **p < 0.01
Fig. 2.
GBE treatment protects against diabetes-induced RGC damage and apoptosis. (A) IF assay was employed to assess the expression of RBPMS in retinal tissues. Scale bars: 50 μm. (B) The TuNEL assay was used to examine the expression of apoptosis in retinal tissues. Scale bars: 50 μm. All experiments included appropriate controls and were repeated at least three times to ensure reproducibility. *p < 0.05, **p < 0.01
Identification and analysis of potential GBE targets for treating DR
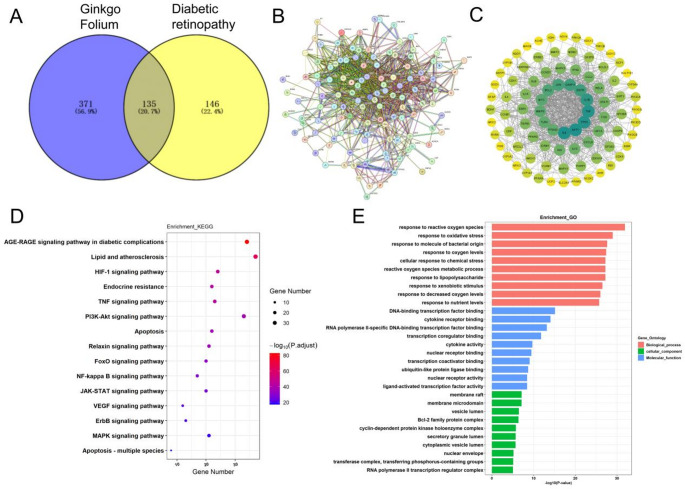
A comprehensive search was conducted using the keywords “Ginkgo Biloba Extract” or “Ginkgo Folium” in the TCSMP and SYMMAP databases to identify potentially active compounds. By applying the selection criteria of an OB greater than 30% and a DL score above 0.18, we Successfully screened for components and their corresponding targets. After eliminating duplicate targets, a total of 506 GBE-related targets were identified (Fig. 3A). For DR, we retrieved targets from Genecards, OMIM, and DisGeNET, identifying 189 common targets across multiple databases as potential DR targets. By intersecting GBE-related targets with potential DR targets, we identified 135 potential targets for GBE in treating DR (Fig. 3A). These targets were input into the STRING database (confidence score > 0.7) to generate a PPI network (Fig. 3B). Topological analysis was conducted using three key metrics: “Degree”, “Closeness”, and “Betweenness”, which measure a node’s importance, centrality, and proximity to other nodes in the PPI network. We selected 93 core targets for GBE in treating DR based on these metrics, with each target having values above the median for all three metrics (Fig. 3C). Finally, KEGG and GO enrichment analyses were performed on the potential targets of GBE in treating DR. Key KEGG pathways included AGE-RAGE, HIF-1, TNF, PI3K-Akt, VEGF, ErbB, MAPK, and multiple apoptosis-related pathways. GO analysis highlighted oxidative stress-related processes, ROS metabolism, and responses to various stimuli, with enrichment in membrane rafts, microdomains, nuclear envelope, and transcription factor/kinase complexes (Fig. 3D, E), suggesting GBE’s potential mechanisms in DR.
Fig. 3.
Identification of potential targets and pathways of GBE in treating diabetic retinopathy. (A) A Venn diagram illustrating the overlap between targets related to GBE and those connected to DR. (B) PPI network of the common targets between GBE and DR. The darker the node color and the larger the area, the higher the Degree value, indicating a greater correlation between GBE and the treatment of DR. (C) Topological analysis of core targets within the PPI network, focusing on the top 93 core targets identified through degree, closeness, and betweenness metrics. (D and E) KEGG and GO enrichment analyses were conducted to assess potential targets and signaling pathways related to GBE’s effects on DR
GBE attenuates DR progression by modulating p53-mediated apoptosis in rat models
Our KEGG pathway enrichment analysis revealed significant associations with apoptosis-related pathways, consistent with previous studies that highlight apoptosis as a critical mechanism in DR progression [37–39]. Further intersection with literature-reported apoptosis-related genes prioritized key targets in our PPI and topological analysis, including TP53, MYC, BCL2, CASP3, CASP9, MDM2, SERPINE1, EGFR, TLR4, PTGS2, and PPARG, which were selected for experimental validation. In DR model rats, RT-qPCR analysis of retinal tissues (n = 3 per group, selected based on HE staining severity) demonstrated significant dysregulation of apoptosis-related genes. Specifically, as illustrated in Fig. 4A, TP53 and MYC expression were significantly higher in the DR model group versus the control group, with effective restoration in the GBE-treated group. This suggests GBE may inhibit the expression of these pro-apoptotic genes. Conversely, the anti-apoptotic gene BCL2 was markedly downregulated in the DR model group, an effect partially counteracted by GBE treatment, indicating a potential protective role of GBE against retinal apoptosis. Additionally, MDM2, a negative regulator of TP53 [40], displayed a pattern the reverse of that for TP53 in the DR model and GBE-intervention group, suggesting a complex interaction between these genes under GBE intervention, potentially modulating the TP53-MDM2 feedback loop and thereby impacting retinal apoptosis. Further WB analysis of retinal tissues (n = 3 per group) demonstrated this point (Fig. 4B). However, BCL-2 and MYC expression exhibited inconsistent trends across samples, with no statistically significant differences observed among groups (Fig. 4B). To gain further insights into the functional impact of GBE on the identified targets, we examined the ubiquitination status of TP53. Ubiquitination is a critical post-translational modification that regulates TP53 stability and activity [41]. Our results revealed a decrease in p53 ubiquitination in the DR model group, which could contribute to the elevated p53 levels observed. Notably, GBE treatment led to an increase in TP53 ubiquitination (Fig. 4C), implying that GBE may modulate TP53 stability and function through this mechanism. This finding supports the notion that GBE’s beneficial effects in DR may be mediated, in part, through its influence on apoptosis-related pathways, specifically by regulating the TP53 ubiquitination to modulate the TP53-MDM2 feedback loop.
Fig. 4.
GBE alleviates DR progression by regulating TP53-dependent apoptosis in rat models. (A) The expressions of TP53, MYC, BCL2, CASP3, CASP9, MDM2, SERPINE1, EGFR, TLR4, PTGS2, and PPARG, were examined in retinal tissue samples from control, model, and GBE-treated rats using RT-qPCR assays. (B) The expressions of MDM2, MYC, TP53, and BCL-2 were examined in retinal tissue samples from control, model, and GBE-treated rats using WB assays. (C) IP analysis of TP53 ubiquitination in retinal tissue. The upper panel showed the IP of TP53 followed by the detection of ubiquitin modification level (IB: UB). The lower panel showed the input levels of TP53. The experiment included appropriate controls and was repeated at least three times to ensure reproducibility. *p < 0.05
Discussion
The present study elucidates the protective effects of GBE on RGCs in a DR rat model. Our findings demonstrate that GBE intervention significantly mitigates diabetes-induced damage to RGCs. This protective effect is particularly pronounced through modulation of the p53-mediated apoptosis pathway, highlighting a critical role for TP53 ubiquitination in GBE’s protective mechanism, thereby offering novel insights into its molecular actions in DR pathogenesis.
The pathogenesis of DR involves complex, multifaceted changes, with neuronal pathology significantly contributing to the decline in visual acuity [12]. Abnormalities in the electroretinogram, visual acuity contrast, visual field range, and color perception all indicate comprehensive visual dysfunction in these patients [42, 43]. The decline in visual parameters can be attributed to the loss of neurons, particularly the axons of RGCs, which are crucial for visual signal relay and possess limited regenerative capacity [44]. Several studies have investigated the transcriptomes of animal retina models in the context of DR, encompassing STZ-induced diabetic rodent models [45–47] and oxygen-induced retinopathy (OIR) models [48, 49]. Additionally, research has focused on quantifying small non-coding RNA in the blood of DR patients [50, 51]. With the emergence of single-cell RNA-sequencing technology, retinal gene expression can now be analyzed at the cellular level [52, 53]. As diabetes prevalence increases, a corresponding rise in DR incidence is expected. However, the current understanding of the molecular etiology and pathways involved in the initiation and progression of DR is limited. In our study, we successfully established a diabetic rat model and induced hyperglycemia, confirmed by significantly elevated FBG levels. The diabetic model rats exhibited retinal pathology, characterized by structural disorganization and thinning of the GCL, indicative of RGC damage. Importantly, GBE-treated diabetic rats exhibited partial preservation of RGC morphology and decreased cellular degeneration, as demonstrated through H&E staining and IF analysis of RBPMS, a renowned biomarker for RGCs [36]. TUNEL staining to detect the apoptotic cells revealed that GBE intervention effectively reversed the stimulatory effect of diabetes on RGC damage.
Apoptosis of retinal cells, particularly RGCs, is a hallmark of DR progression, driven by chronic hyperglycemia-induced oxidative stress and inflammatory cascades [54]. Our findings highlight that GBE intervention significantly mitigates diabetes-induced apoptosis in RGCs. GBE, a popular dietary supplement, exhibits antioxidant, free radical-scavenging, membrane-stabilizing, platelet-activating factor-inhibiting, vasodilatory, and metabolic regulatory properties [55]. Increasing clinical studies focus on its application in cardiovascular disease, peripheral vascular disease (PVD), and diabetic vascular complications [28, 56, 57]. As a promising therapeutic for cardiovascular and ischaemic diseases, GBE offers vascular protection through multifaceted mechanisms. However, the mechanism of GBE in DR, a typical diabetic vascular complication, remains to be developed. Our bioinformatics analysis identified a total of 506 GBE-related targets, which overlapped with 189 potential DR targets, resulting in 135 common targets. This intersection approach allowed us to prioritize targets that are both relevant to DR and Susceptible to GBE intervention. Notably, topological analysis of the PPI network revealed 93 core targets for GBE in treating DR, with MDM2, TP53, BCL2, and MYC standing out as key nodes due to their vital role in apoptosis. The upregulation of TP53 in diabetic retinal tissues aligns with its established role as a tumor suppressor that promotes apoptosis in response to cellular stress [58, 59]. Elevated TP53 levels in the DR model group likely reflect sustained DNA damage and metabolic dysregulation in hyperglycemic conditions, triggering RGC loss. Notably, GBE administration significantly attenuated TP53 overexpression, suggesting its capacity to counteract stress-induced apoptotic signaling. This finding corroborates prior evidence that natural compounds targeting TP53 stabilization can ameliorate DR pathology [60, 61]. A key finding in this study is GBE’s ability to enhance TP53 ubiquitination, promoting its degradation. The scientific community recognizes that TP53 triggers apoptosis by regulating gene expression when cellular damage is irreparable [62]. However, ubiquitination of TP53 can inhibit its activity, subsequently affecting the expression of its downstream targets [41]. In the diabetic retinas of our rat model, lower ubiquitination likely upregulates TP53’s expression, exacerbating pro-apoptotic signaling. The observed restoration of ubiquitination by GBE implies a direct or indirect modulation of the MDM2-TP53 axis, where MDM2 acts as an E3 ubiquitin ligase for TP53 [63]. Although MDM2 expression trends were unstable in our WB analysis, the functional recovery of qPCR results and TP53 ubiquitination suggests that GBE may stabilize MDM2-TP53 interactions or enhance ubiquitin ligase activity, even under diabetic conditions. These findings align with previous studies that have implicated apoptosis as a crucial mechanism in the pathogenesis of DR [54]. The regulation of TP53 ubiquitination by GBE suggests a novel therapeutic strategy for mitigating DR progression. By modulating TP53-mediated apoptosis, GBE could potentially prevent excessive death of RGCs and maintain retinal function, thereby offering a promising approach for managing DR. While our findings emphasize TP53 ubiquitination as a key mechanism, several limitations warrant consideration. Small sample size (n = 3/group) for WB analysis may reduce statistical power, especially for variable proteins. We acknowledge the Limitation regarding clinical relevance. While the STZ-induced diabetes model is widely used in basic research due to its well-established role in elucidating early pathophysiological mechanisms of diabetes, which are shared across both type 1 and type 2 diabetes [29–33], we must recognize that this model primarily represents type 1 diabetes. In contrast, the majority of clinical cases occur in patients with type 2 diabetes. We recognize the limitation of not measuring direct visual function. Notably, DR, the predominant microvascular complication of diabetes and a major cause of blindness among young adults, was traditionally treated by managing systemic factors and using laser photocoagulation. However, the introduction of anti-VEGF agents (including Ranibizumab [64], aflibercept, and Bevacizumab [65]), over the past decade has transformed DR treatment. While our study enhances the understanding of GBE’s molecular mechanisms and highlights TP53 as a promising therapeutic target for DR intervention, the absence of a positive control group using drugs like ranibizumab limits direct comparison. We recognize that evaluating the comparative efficacy and safety of GBE versus standard treatments necessitates randomized controlled trials with direct head-to-head comparisons, which will be a key focus of our future research. Additionally, the study focuses on TP53-driven apoptosis, yet GBE’s bioactivity may involve broader pathways, such as oxidative stress and inflammation, as indicated by KEGG enrichment (AGE-RAGE, HIF-1, and TNF pathways). Future work should validate these pathways, explore crosstalk, and validate results in advanced DR models or human retinal cells. More functional assessments of vision will be performed in animals, e.g., electrophysiological (ERG) or behavioral testing, to correlate molecular changes with functional benefits. What’s more, while our 30-day intervention, consistent with established models for initial efficacy screening in early-stage DR [66], was sufficient to detect significant improvements in key pathological markers (Figs. 1B, amp and C and 2), a critical focus for future research will be to investigate the long-term effects and systematic toxicology profile of GBE prior to its clinical translation [67]. Such extended studies are essential to comprehensively evaluate its influence on the progression of diabetic retinopathy, particularly regarding later-stage pathological features and long-term safety.
Conclusion
In summary, this study demonstrates that GBE attenuates DR progression by targeting TP53-mediated apoptosis, primarily through the regulation of TP53 ubiquitination and degradation. These findings advance our understanding of GBE’s molecular mechanisms and underscore TP53 as a therapeutic node for DR intervention.
Acknowledgements
This work was supported by Zhejiang Provincial Medical and Health Science and Technology Plan (Grant No. 2023RC122).
Author contributions
J.Y.: Conceptualization; Data curation; Investigation; Methodology; Writing – original draft. Y.S.: Conceptualization; Investigation; Formal analysis; Writing – review and editing. All authors reviewed the manuscript.
Funding
This work was supported by Zhejiang Provincial Medical and Health Science and Technology Plan [Grant No. 2023RC122].
Data availability
All data generated or analyzed during this study are shown within this article.
Declarations
Competing interests
The authors declare no competing interests.
Ethical approval
The animal experiments were conducted in accordance with the “Guiding Principles in the Care and Use of Animals” (China), and approved by Shengyuan Life Sciences and Technology (Anji) Co., Ltd. (approval number: 2025-002).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.You Y, Gupta VK, Li JC, Klistorner A, Graham SL (2013) Optic neuropathies: characteristic features and mechanisms of retinal ganglion cell loss. Rev Neurosci 24:301–321 [DOI] [PubMed] [Google Scholar]
- 2.Xu T, Wang B, Liu H, Wang H, Yin P, Dong W, Li J, X Wang Y, Yusufu M, Briant P, Reinig N, Ashbaugh C, Adelson J, Vos T, Bourne R, Wang N, Zhou M (2020) Prevalence and causes of vision loss in China from 1990 to 2019: findings from the global burden of disease study 2019. Lancet Public Health 5:e682–e691 [DOI] [PubMed] [Google Scholar]
- 3.Lyu J, Mu X (2021) Genetic control of retinal ganglion cell genesis. Cell Mol Life Sci 78:4417–4433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Risner ML, Pasini S, McGrady NR, Calkins DJ (2022) Bax contributes to retinal ganglion cell dendritic degeneration during glaucoma. Mol Neurobiol 59:1366–1380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levkovitch-Verbin H (2015) Retinal ganglion cell apoptotic pathway in glaucoma: initiating and downstream mechanisms. Prog Brain Res 220:37–57 [DOI] [PubMed] [Google Scholar]
- 6.Wen YT, Huang CW, Liu CP, Chen CH, Tu CM, Hwang CS, Chen YH, Chen WR, Lin KL, Ho YC, Chen TC, Tsai RK (2021) Inhibition of retinal ganglion cell loss by a novel ROCK inhibitor (E212) in ischemic optic nerve injury via antioxidative and anti-inflammatory actions. Invest Ophthalmol Vis Sci 62:21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun MH, Chen KJ, Sun CC, Tsai RK (2022) Protective effect of Pioglitazone on retinal ganglion cells in an experimental mouse model of ischemic optic neuropathy. Int J Mol Sci. 10.3390/ijms24010411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ju WK, Perkins GA, Kim KY, Bastola T, Choi WY, Choi SH (2023) Glaucomatous optic neuropathy: mitochondrial dynamics, dysfunction and protection in retinal ganglion cells. Prog Retin Eye Res 95:101136 [DOI] [PubMed] [Google Scholar]
- 9.Au NPB, Ma CHE (2022) Neuroinflammation, microglia and implications for retinal ganglion cell survival and axon regeneration in traumatic optic neuropathy. Front Immunol 13:860070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cloete L (2022) Diabetes mellitus: an overview of the types, symptoms, complications and management. Nurs Standard (Royal Coll Nurs (Great Britain): 1987) 37:61–66 [DOI] [PubMed] [Google Scholar]
- 11.Aiello LM (2003) Perspectives on diabetic retinopathy. Am J Ophthalmol 136:122–135 [DOI] [PubMed] [Google Scholar]
- 12.Cheung N, Mitchell P, Wong TY (2010) Diabetic retinopathy. Lancet (London England) 376:124–136 [DOI] [PubMed] [Google Scholar]
- 13.Yang S, Zhang J, Chen L (2020) The cells involved in the pathological process of diabetic retinopathy. Biomed Pharmacother 132:110818 [DOI] [PubMed] [Google Scholar]
- 14.Giurdanella G, Lupo G, Gennuso F, Conti F, Furno DL, Mannino G, Anfuso CD, Drago F, Salomone S, Bucolo C (2020) Activation of the VEGF-A/ERK/PLA2 axis mediates early retinal endothelial cell damage induced by high glucose: new insight from an in vitro model of diabetic retinopathy. Int J Mol Sci. 10.3390/ijms21207528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song S, Yu X, Zhang P, Dai H (2020) Increased levels of cytokines in the aqueous humor correlate with the severity of diabetic retinopathy. J Diabetes Complications 34:107641 [DOI] [PubMed] [Google Scholar]
- 16.Tu Y, Li L, Zhu L, Guo Y, Du S, Zhang Y, Wang Z, Zhang Y, Zhu M (2021) Geniposide Attenuates Hyperglycemia-Induced Oxidative Stress and Inflammation by Activating the Nrf2 Signaling Pathway in Experimental Diabetic Retinopathy. Oxidative medicine and cellular longevity 2021, 9247947 [DOI] [PMC free article] [PubMed]
- 17.Xu Y, Peng Y, Wu X, Ni F, Sun D, Zhang P, Yang Y, Yan M, Mi J, Tian G (2024) VEGF-B prevents chronic hyperglycemia-induced retinal vascular leakage by regulating the CDC42-ZO1/VE-cadherin pathway. FASEB J 38:e70019 [DOI] [PubMed] [Google Scholar]
- 18.Midena E, Pilotto E (2017) Emerging insights into pathogenesis. Dev Ophthalmol 60:16–27 [DOI] [PubMed] [Google Scholar]
- 19.Wu H, Xu G, Liao Y, Ren H, Fan J, Sun Z, Zhang M (2012) Supplementation with antioxidants attenuates transient worsening of retinopathy in diabetes caused by acute intensive insulin therapy. Graefe’s Archive Clin Experimental Ophthalmol = Albrecht Von Graefes Archiv Fur Klinische Und Experimentelle Ophthalmologie 250:1453–1458 [DOI] [PubMed] [Google Scholar]
- 20.Bucolo C, Leggio GM, Drago F, Salomone S (2012) Eriodictyol prevents early retinal and plasma abnormalities in streptozotocin-induced diabetic rats. Biochem Pharmacol 84:88–92 [DOI] [PubMed] [Google Scholar]
- 21.Bucolo C, Marrazzo G, Platania CB, Drago F, Leggio GM, Salomone S (2013) Fortified extract of red berry, Ginkgo biloba, and white Willow bark in experimental early diabetic retinopathy. J Diabetes Res 2013:432695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilieva I, Ohgami K, Shiratori K, Koyama Y, Yoshida K, Kase S, Kitamei H, Takemoto Y, Yazawa K, Ohno S (2004) The effects of Ginkgo biloba extract on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp Eye Res 79:181–187 [DOI] [PubMed] [Google Scholar]
- 23.Lejri I, Grimm A, Eckert A (2019) Ginkgo biloba extract increases neurite outgrowth and activates the Akt/mTOR pathway. PLoS ONE 14:e0225761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhein V, Giese M, Baysang G, Meier F, Rao S, Schulz KL, Hamburger M, Eckert A (2010) Ginkgo biloba extract ameliorates oxidative phosphorylation performance and rescues abeta-induced failure. PLoS ONE 5:e12359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshikawa T, Naito Y, Kondo M (1999) Ginkgo biloba leaf extract: review of biological actions and clinical applications. Antioxid Redox Signal 1:469–480 [DOI] [PubMed] [Google Scholar]
- 26.Xia C, Zhou M, Dong X, Zhao Y, Jiang M, Zhu G, Zhang Z (2024) Ginkgo biloba extract inhibits hippocampal neuronal injury caused by mitochondrial oxidative stress in a rat model of Alzheimer’s disease. PLoS ONE 19:e0307735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh SK, Srivastav S, Castellani RJ, Plascencia-Villa G, Perry G (2019) Neuroprotective and antioxidant effect of Ginkgo Biloba extract against AD and other neurological disorders. Neurotherapeutics: J Am Soc Experimental Neurother 16:666–674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martínez-Solís I, Acero N, Bosch-Morell F, Castillo E, González-Rosende ME, Muñoz-Mingarro D, Ortega T, Sanahuja MA, Villagrasa V (2019) Neuroprotective potential of Ginkgo biloba in retinal diseases. Planta Med 85:1292–1303 [DOI] [PubMed] [Google Scholar]
- 29.Zhang S, Ma X, Zhang L, Sun H, Liu X (2017) Capsaicin reduces blood glucose by increasing insulin levels and glycogen content better than capsiate in Streptozotocin-induced diabetic rats. J Agric Food Chem 65:2323–2330 [DOI] [PubMed] [Google Scholar]
- 30.Yang B, Luo Y, Wei X, Kan J (2022) Polysaccharide from Hovenia dulcis (Guaizao) improves pancreatic injury and regulates liver glycometabolism to alleviate STZ-induced type 1 diabetes mellitus in rats. Int J Biol Macromol 214:655–663 [DOI] [PubMed] [Google Scholar]
- 31.Hu T, Wu Q, Yao Q, Yu J, Jiang K, Wan Y, Tang Q (2023) PRDM16 exerts critical role in myocardial metabolism and energetics in type 2 diabetes induced cardiomyopathy. Metabolism 146:155658 [DOI] [PubMed] [Google Scholar]
- 32.Olivares AM, Althoff K, Chen GF, Wu S, Morrisson MA, DeAngelis MM, Haider N (2017) Animal models of diabetic retinopathy. Curr Diab Rep 17:93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Q, Wang P, Gong Y, Xu M, Wang M, Luan R, Liu J, Li X, Shao Y (2024) α-Klotho prevents diabetic retinopathy by reversing the senescence of macrophages. Cell Commun Signal 22(CCS):449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu JH, Li YN, Chen AQ, Hong CD, Zhang CL, Wang HL, Zhou YF, Li PC, Wang Y, Mao L, Xia YP, He QW, Jin HJ, Yue ZY, Hu B (2020) Inhibition of Sema4D/PlexinB1 signaling alleviates vascular dysfunction in diabetic retinopathy. EMBO Mol Med 12:e10154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin Y, Xu R, Ning L, Yu Z (2023) Bergenin alleviates diabetic retinopathy in STZ-induced rats. Appl Biochem Biotechnol 195:5299–5311 [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez AR, de Sevilla Müller LP, Brecha NC (2014) The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. J Comp Neurol 522:1411–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Li HY, Shao J, Zhu L, Xie TH, Cai J, Wang W, Cai MX, Wang ZL, Yao Y, Wei TT (2022) GRP75 modulates endoplasmic reticulum-mitochondria coupling and accelerates Ca(2+)-dependent endothelial cell apoptosis in diabetic retinopathy. Biomolecules. 10.3390/biom12121778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang X, Su D, Wei D, Chen X, Hu Y, Li S, Zhang Y, Ma X, Hu S, Sun Z (2024) Role of MST2/YAP1 signaling pathway in retinal cells apoptosis and diabetic retinopathy. Toxicol Appl Pharmacol 484:116885 [DOI] [PubMed] [Google Scholar]
- 39.Adamiec-Mroczek J, Zając-Pytrus H, Misiuk-Hojło M (2015) Caspase-dependent apoptosis of retinal ganglion cells during the development of diabetic retinopathy. Adv Clin Experimental Medicine: Official Organ Wroclaw Med Univ 24:531–535 [DOI] [PubMed] [Google Scholar]
- 40.Koo N, Sharma AK, Narayan S (2022) Therapeutics targeting p53-MDM2 interaction to induce cancer cell death. Int J Mol Sci. 10.3390/ijms23095005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu J, Guan D, Dong M, Yang J, Wei H, Liang Q, Song L, Xu L, Bai J, Liu C, Mao J, Zhang Q, Zhou J, Wu X, Wang M, Cong YS (2020) Ufmylation maintains tumour suppressor p53 stability by antagonizing its ubiquitination. Nat Cell Biol 22:1056–1063 [DOI] [PubMed] [Google Scholar]
- 42.Lieth E, Gardner TW, Barber AJ, Antonetti DA (2000) Retinal neurodegeneration: early pathology in diabetes. Clin Exp Ophthalmol 28:3–8 [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Wang N, Barile GR, Bao S, Gillies M (2013) Diabetic retinopathy: neuron protection as a therapeutic target. Int J Biochem Cell Biol 45:1525–1529 [DOI] [PubMed] [Google Scholar]
- 44.Fischer D, He Z, Benowitz LI (2004) Counteracting the Nogo receptor enhances optic nerve regeneration if retinal ganglion cells are in an active growth state. J Neuroscience: Official J Soc Neurosci 24:1646–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kandpal RP, Rajasimha HK, Brooks MJ, Nellissery J, Wan J, Qian J, Kern TS, Swaroop A (2012) Transcriptome analysis using next generation sequencing reveals molecular signatures of diabetic retinopathy and efficacy of candidate drugs. Mol Vis 18:1123–1146 [PMC free article] [PubMed] [Google Scholar]
- 46.He K, Lv W, Zhang Q, Wang Y, Tao L, Liu D (2015) Gene set enrichment analysis of pathways and transcription factors associated with diabetic retinopathy using a microarray dataset. Int J Mol Med 36:103–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu YJ, Lian ZY, Liu G, Zhou HY, Yang HJ (2016) RNA sequencing reveals retinal transcriptome changes in STZ-induced diabetic rats. Mol Med Rep 13:2101–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rojo Arias JE, Economopoulou M, Juárez López DA, Kurzbach A, Au Yeung KH, Englmaier V, Merdausl M, Schaarschmidt M, Ader M, Morawietz H, Funk RHW, Jászai J (2020) VEGF-Trap is a potent modulator of vasoregenerative responses and protects dopaminergic amacrine network integrity in degenerative ischemic neovascular retinopathy. J Neurochem 153:390–412 [DOI] [PubMed] [Google Scholar]
- 49.Zasada M, Madetko-Talowska A, Revhaug C, Rognlien AGW, Baumbusch LO, Książek T, Szewczyk K, Grabowska A, Bik-Multanowski M, Józef Pietrzyk J, Kwinta P, Saugstad OD (2020) Short- and long-term impact of hyperoxia on the blood and retinal cells’ transcriptome in a mouse model of oxygen-induced retinopathy. Pediatr Res 87:485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liang Z, Gao KP, Wang YX, Liu ZC, Tian L, Yang XZ, Ding JY, Wu WT, Yang WH, Li YL, Zhang ZB, Zhai RH (2018) RNA sequencing identified specific circulating MiRNA biomarkers for early detection of diabetes retinopathy. Am J Physiol Endocrinol Metab 315:E374-e385 [DOI] [PubMed] [Google Scholar]
- 51.Li Z, Dong Y, He C, Pan X, Liu D, Yang J, Sun L, Chen P, Wang Q (2019) RNA-seq revealed novel non-proliferative retinopathy specific circulating miRNAs in T2DM patients. Front Genet 10:531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lukowski SW, Lo CY, Sharov AA, Nguyen Q, Fang L, Hung SS, Zhu L, Zhang T, Grünert U, Nguyen T, Senabouth A, Jabbari JS, Welby E, Sowden JC, Waugh HS, Mackey A, Pollock G, Lamb TD, Wang PY, Hewitt AW, Gillies MC, Powell JE, Wong RC (2019) A single-cell transcriptome atlas of the adult human retina. EMBO J 38:e100811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Menon M, Mohammadi S, Davila-Velderrain J, Goods BA, Cadwell TD, Xing Y, Stemmer-Rachamimov A, Shalek AK, Love JC, Kellis M, Hafler BP (2019) Single-cell transcriptomic atlas of the human retina identifies cell types associated with age-related macular degeneration. Nat Commun 10:4902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kang Q, Yang C (2020) Oxidative stress and diabetic retinopathy: molecular mechanisms, pathogenetic role and therapeutic implications. Redox Biol 37:101799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tian J, Liu Y, Chen K (2017) Ginkgo biloba extract in vascular protection: molecular mechanisms and clinical applications. Curr Vasc Pharmacol 15:532–548 [DOI] [PubMed] [Google Scholar]
- 56.Peng Y, Chen Q, Xue YH, Jin H, Liu S, Du MQ, Yao SY (2024) Ginkgo biloba and its chemical components in the management of Alzheimer’s disease. Am J Chin Med 52:625–666 [DOI] [PubMed] [Google Scholar]
- 57.Evans JR (2013) Ginkgo biloba extract for age-related macular degeneration. The Cochrane database of systematic reviews 2013, Cd001775 [DOI] [PubMed]
- 58.Aubrey BJ, Strasser A, Kelly GL (2016) Tumor-suppressor functions of the TP53 pathway. Cold Spring Harb Perspect Med. 10.1101/cshperspect.a026062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Aubrey BJ, Kelly GL, Janic A, Herold MJ, Strasser A (2018) How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ 25:104–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yao H, Xin D, Zhan Z, Li Z (2021) Network Pharmacology-Based Approach to Comparatively Predict the Active Ingredients and Molecular Targets of Compound Xueshuantong Capsule and Hexuemingmu Tablet in the Treatment of Proliferative Diabetic Retinopathy. Evidence-based complementary and alternative medicine: eCAM 2021, 6642600 [DOI] [PMC free article] [PubMed]
- 61.Zhou Y, Fan G, Zhang Y, Xu L (2022) Identification of potential molecular targets and active ingredients of Mingmu Dihuang pill for the treatment of diabetic retinopathy based on network Pharmacology. Biomed Res Int 2022. 2896185, doi: 10.1155/2022/2896185. eCollection 2022. [DOI] [PMC free article] [PubMed]
- 62.Vaddavalli PL, Schumacher B (2022) The p53 network: cellular and systemic DNA damage responses in cancer and aging. Trends Genet 38:598–612 [DOI] [PubMed] [Google Scholar]
- 63.Zhou J, Kryczek I, Li S, Li X, Aguilar A, Wei S, Grove S, Vatan L, Yu J, Yan Y, Liao P, Lin H, Li J, Li G, Du W, Wang W, Lang X, Wang W, Wang S, Zou W (2021) The ubiquitin ligase MDM2 sustains STAT5 stability to control T cell-mediated antitumor immunity. Nat Immunol 22:460–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chatziralli I (2021) Ranibizumab for the treatment of diabetic retinopathy. Expert Opin Biol Ther 21:991–997 [DOI] [PubMed] [Google Scholar]
- 65.Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, Brucker AJ, Ferris FL, Hampton GR, Jhaveri C, Melia M, Beck RW (2016) Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology 123:1351–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Su Q, Dong J, Zhang D, Yang L, Roy R (2022) Protective effects of the bilobalide on retinal oxidative stress and inflammation in Streptozotocin-induced diabetic rats. Appl Biochem Biotechnol 194:6407–6422 [DOI] [PubMed] [Google Scholar]
- 67.Huang SY, Jeng C, Kao SC, Yu JJ, Liu DZ (2004) Improved haemorrheological properties by Ginkgo biloba extract (Egb 761) in type 2 diabetes mellitus complicated with retinopathy. Clin Nutr 23:615–621 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are shown within this article.