Abstract
Pyridoxine-dependent epilepsy (PDE) is a rare neurometabolic disorder of lysine catabolism caused by bi-allelic variants in ALDH7A1. This enzyme deficiency leads to accumulation of neurotoxic metabolites, pyridoxal-phosphate inactivation, and consequently severe neurological symptoms. Current treatments, including vitamin B6 supplementation and lysine-restricted diets, partially alleviate seizures and intellectual disability but are not curative. To explore underlying mechanisms and potential therapies, we generated patient-derived human induced pluripotent stem cell (hiPSC) lines that were subsequently differentiated into astrocytes, the primary source of ALDH7A1 in the brain and key regulators of metabolic homeostasis. Metabolomic analyses confirmed elevated PDE biomarkers, and RNA sequencing revealed gene expression changes consistent with increased oxidative stress. Oxidative damage was validated by markers of DNA oxidation, increased reactive oxygen species (ROS) levels, and lipid peroxidation. In addition, dysregulated oxygen consumption rates suggested mitochondrial dysfunction in PDE astrocytes. Notably, these pathological phenotypes were alleviated by downregulating alpha-aminoadipic semialdehyde synthase (AASS), the first enzyme of the lysine catabolism, by using CRISPR-Cas9 editing or antisense oligonucleotides (AONs). This demonstrates that lysine catabolism underlies these phenotypes and highlights the therapeutic potential of AON therapy targeting AASS to reduce neurotoxic metabolite accumulation. These findings provide a promising strategy for developing targeted treatments for PDE and other rare neurometabolic disorders.
Keywords: MT: Oligonucleotides: Therapies and Applications, ALDH7A1, AASS, induced pluripotent stem cells, astrocytes, human disease modeling, antisense oligonucleotides, metabolic diseases, lysine metabolism disorders
Graphical abstract

Using patient-derived astrocytes, Schuurmans and colleagues show that pyridoxine-dependent epilepsy (PDE) causes toxic metabolite buildup, oxidative stress, and mitochondrial dysfunction. These phenotypes were (partially) rescued by downregulating AASS upstream in the lysine pathway, providing first proof of concept for a translatable therapy for patients with PDE and highlighting potential for other neurometabolic disorders.
Introduction
Pyridoxine-dependent epilepsy (PDE) is a rare neurometabolic disorder of lysine catabolism, with an estimated incidence around 1:65,000.1 PDE is characterized by recurrent perinatal-onset seizures that are resistant to conventional anticonvulsant drugs but instead show remarkable response to pyridoxine administration.2 In addition to epilepsy, more than 75% of patients with PDE exhibit developmental delay and moderate-to-severe intellectual disability,3 in some cases combined with structural brain abnormalities including hypoplasia of the corpus callosum and/or cerebellum.4,5 PDE is caused by autosomal recessive pathogenic variants in ALDH7A1. The encoded enzyme is responsible for the conversion of 2-aminoadipic-6-semialdehyde (α-AASA) to 2-aminoadipic acid (AAA) in the lysine degradation pathway. ALDH7A1 enzyme deficiency therefore results in the accumulation of neurotoxic metabolites including α-AASA, Δ1-piperideine-6-L-carboxylate (P6C), and pipecolic acid (PA). Excessive P6C is known to complex with pyridoxal phosphate (PLP), resulting in its lowered availability and therefore PLP inactivation.1 PLP is the active form of vitamin B6 (pyridoxine) acting as an important cofactor for several enzymes in the brain, including enzymes involved in GABA synthesis.6 PLP inactivation is therefore thought to underlie the pyridoxine-responsive seizures. Astrocytes are the main cell type expressing ALDH7A1 in the brain.7,8 Since the accumulation of toxic lysine degradation intermediates primarily occurs in these cells, they are thought to play a crucial role in the disease mechanisms underlying PDE,2,7,9,10,11,12 but the exact mechanisms remain unclear.
Currently, the only available treatment strategies for PDE include vitamin B6 (pyridoxine) supplementation for seizure control combined with dietary interventions aimed at reducing toxic metabolite accumulation by lysine reduction and arginine supplementation. Arginine is included as a competitive inhibitor for lysine to cross the blood-brain barrier as lysine and arginine use the same cationic transporter.13 However, these dietary interventions have limited efficacy as lysine is an essential amino acid that cannot be completely removed. Thus, there is an unmet need to develop novel therapeutic strategies as lysine reduction does not eliminate but at best mitigates neurologic impairments.14
Upstream enzyme inhibition to prevent buildup of toxic metabolites has been successful for other inherited metabolic disorders (IMDs) such as tyrosinemia type I.15 A similar strategy could provide an alternative therapy for PDE instead of current dietary interventions. Alpha-aminoadipic semialdehyde synthase (AASS) is a bifunctional enzyme, consisting of the lysine ketoglutarate reductase (LKR) domain and saccharopine dehydrogenase (SDH) domain, catalyzing the first and second step, respectively, of the saccharopine pathway of lysine catabolism. Importantly, near-complete AASS deficiency in humans, in particular when the LKR domain is affected, is referred to as hyperlysinemia type I and leads to lysine accumulation, which is associated with none or mild clinically relevant phenotypes.16,17,18,19 This provides most direct evidence that AASS could be a safe target for therapeutic inhibition. Given that AASS works upstream of ALDH7A1 in the lysine pathway, AASS is considered a potential target for therapeutic inhibition to prevent the cerebral accumulation of toxic lysine metabolites in PDE. Several preclinical studies already showed the effectiveness of inhibiting AASS to treat glutaric aciduria type I (GA1), another disorder of lysine catabolism,20,21 and during the revision of this manuscript, evidence through knockout of AASS using mouse models has emerged, also supporting this strategy for PDE.22,23 A promising strategy for partial inhibition of AASS is antisense oligonucleotides (AONs). These are small nucleic acid molecules that bind complementarily to the pre-mRNA or mRNA and thereby, among other functions, can modulate degradation of mRNA transcripts.24 Several AON strategies have already demonstrated promising results in clinical trials for other inherited diseases, particularly those affecting the eye or brain, due to the benefits of local delivery.25
Currently available PDE model systems include Aldh7a1−/− mice and zebrafish, both recapitulating some of the typical PDE characteristics, including the pyridoxine-dependent seizures.2,26 Although these PDE animal models have improved our understanding of the PDE disease mechanisms, the use of animal models also has limitations including functional and genomic differences.27,28 Human induced pluripotent stem cells (hiPSCs) offer opportunities for research into human and cell-type-specific pathophysiological mechanisms underlying PDE and allow us to test new genetic therapeutic strategies for PDE in a patient’s context.
To this end, we developed an isogenic ALDH7A1−/− (ALDH7A1 knockout [KO]) hiPSC line and generated three PDE patient-derived hiPSC lines. Considering the enriched expression of ALDH7A1 in astrocytes compared to other neuronal cell types and their previously suggested role in the pathophysiology of PDE, we differentiated the ALDH7A1 KO and PDE patient-derived hiPSC lines into astrocytes. Our data show that the ALDH7A1 KO as well as the PDE patient astrocytes show elevated PDE biomarkers, increased oxidative stress, and heightened oxygen consumption rates (OCRs), indicative of mitochondrial dysfunction. Notably, we demonstrated that partial inhibition of AASS, the first enzyme in lysine catabolism, using AONs effectively ameliorated these metabolic abnormalities and normalized OCRs.
Results
PDE patient-derived astrocytes show elevated PDE biomarkers
To investigate the disease mechanism underlying PDE, we generated hiPSC lines from patients with PDE29 and an isogenic hiPSC ALDH7A1 KO line.30 Fibroblasts from three PDE patients (P1–P3) were reprogrammed toward hiPSCs, all harboring the most commonly reported ALDH7A1 homozygous c.1279G>C (p.Glu427Gln) variant (Figure 1A). We used CRISPR-Cas9 genome editing in a healthy control line (C1) to generate an isogenic ALDH7A1 KO line (Figure 1B). As expected, western blotting revealed complete loss of ALDH7A1 protein levels in the isogenic ALDH7A1 KO line, consistent with the absence of ALDH7A1 expression at RNA level as previously shown by quantitative PCR (qPCR).30 To further minimalize potential effects of genetic background, we also included four independent control lines (C1–C4) for comparison.
Figure 1.
Metabolic characterization of ALDH7A1 KO and PDE patient-derived hiPSC astrocytes
(A) Schematic overview of the four control and three PDE-patient hiPSC lines used in this study carrying the pathogenic ALDH7A1 variant c.1279G>C (p.Glu427Gln) in homozygosis. (B) Schematic overview of ALDH7A1 KO (KO) hiPSC line and C1 including previously published western blot for ALDH7A1 and GAPDH protein levels for the KO and its control line.30 (C) Expression of ALDH7A1 relative to ACTB by RT-PCR in hiPSCs, hiPSC-derived astrocytes, and hiPSC-derived neurons from 14, 21, 28, 35, and 42 days in vitro (DIV). (D) Schematic representation of the protocol to differentiate hiPSCs toward astrocytes. (E) Representative images of immunostaining of GFAP (magenta), CD44 (white), GLUD1 (magenta), and ALDH1L1 (white) in DIV 35 C1 and KO astrocytes. All pictures were taken at the same magnification (scale bars, 50 μm). (F) Schematic representation of ALDH7A1 deficiency in the lysine pathway, including the corresponding PDE biomarkers. The fold change (FC) of the normalized intensity (peak area) of P6C, PA, and AAA measured via NGMS in KO and C1 astrocytes is shown in the top three graphs. n = 5/2 for C1; n = 5/2 for KO. Statistically significant differences were tested through unpaired t test. In the bottom three graphs, the FC of the normalized intensity of the same biomarkers is shown for the three PDE patient lines as well as the four control astrocyte lines (C1–C4). n = 19/2 for C1–C4; n = 5/2 for P1; n = 5/2 for P2; n = 6/2 for P3. Statistically significant differences for P6C and PA were tested through one-way ANOVA and Dunnett’s multiple comparison test, while Kruskal-Wallis test combined with Dunn’s multiple comparison test was used for the AAA biomarker. Data are shown as mean values with standard deviation (mean ± SD) For all graphs in this figure, statistical significance is indicated by ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005, ∗∗∗∗p < 0.0001. Exact p value per condition is provided in Table S1.
Astrocytes are thought to play a key role in the pathophysiology of PDE,2,7,11 especially as the expression of ALDH7A1 is enriched in astrocytes compared to neurons.7 We confirmed that ALDH7A1 expression was higher in hiPSC astrocytes compared to hiPSCs and hiPSC neurons, in which the expression of ALDH7A1 rapidly decreases during differentiation (Figures 1C and S1A). Given these findings, we considered hiPSC-derived astrocytes to be most suitable for further studying PDE disease mechanisms. We differentiated the hiPSC lines toward astrocytes (Figure 1D), as described recently.31 All hiPSC lines differentiated toward astrocytes, without observable differences between cell lines. At days in vitro 35, the astrocytes showed expression of typical astrocyte markers including GFAP, CD44, ALDH1L1, and GLUD1 (Figures 1E and S1B). In addition, the hiPSC-derived astrocyte cultures showed typical monoculture astrocyte morphology.
To investigate the metabolic phenotype of the PDE astrocytes, we measured several intracellular PDE biomarkers, including P6C, PA, and AAA, under basal conditions using next-generation metabolic screening (NGMS, Figures 1F and S2). As expected, P6C and PA were significantly increased in the ALDH7A1 KO astrocytes compared to control, while AAA was significantly decreased. A similar overall metabolic profile was observed in PDE patient astrocytes, though P6C and PA levels were only significantly higher in P3 compared to control. Whereas AAA levels showed considerable variability in the control group, AAA levels were significantly lower in P2 astrocytes. Overall, the metabolic profiles of ALDH7A1 KO and PDE patient astrocytes align with the metabolic phenotypes observed in patients with PDE in vivo.32,33,34,35
Increased oxidative stress and impaired oxygen response in PDE astrocytes
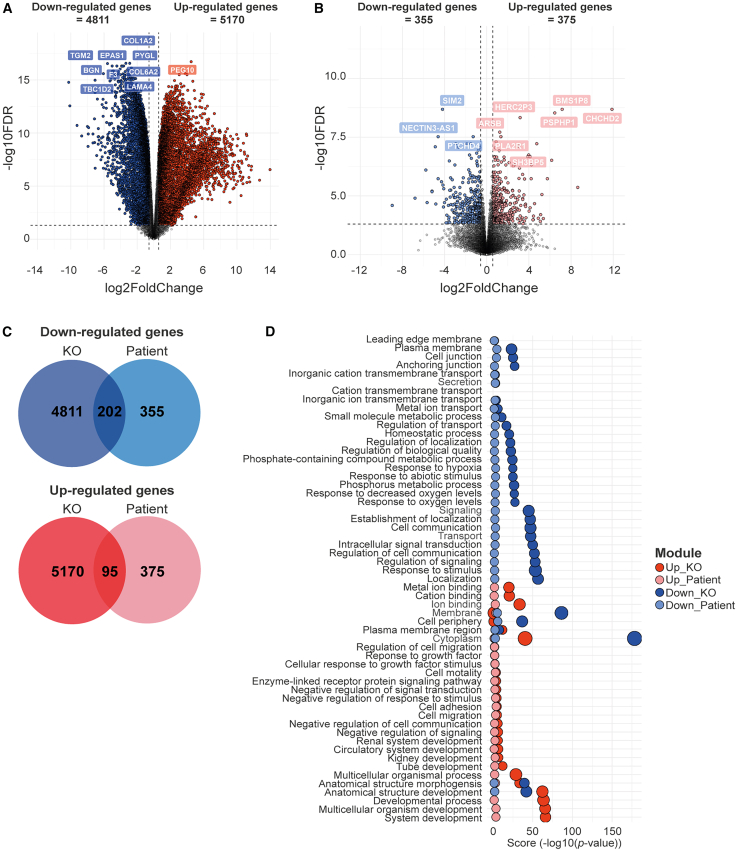
To explore for the disease mechanisms underlying PDE, we analyzed transcriptional differences in a subset of selected PDE astrocyte lines using bulk RNA sequencing: we selected two controls (C1 and C2), two PDE patient lines (P1 and P2), and ALDH7A1 KO astrocytes. Principal component analysis confirmed transcriptional changes across hiPSC astrocyte cultures, clearly separating the different lines (Figure S3A). Subsequent differential gene expression analysis was conducted independently for the ALDH7A1 KO astrocytes with respect to their isogenic control (Figure 2A) and for the PDE patient astrocytes in comparison to pooled controls (Figure 2B). This analysis revealed a total of 4,811 downregulated and 5,170 upregulated genes (Figure 2C) in the ALDH7A1 KO astrocytes compared to C1 astrocytes. The number of differentially expressed genes (DEGs) in PDE patient astrocytes versus controls was lower, with 355 downregulated and 375 upregulated genes (Figure 2C). Among these transcriptomic profiles, we sought to identify pathways most relevant to PDE. We performed gene ontology (GO) enrichment analysis in both the isogenic pair and PDE patient astrocytes, focusing on shared pathways affected across all PDE astrocyte lines (Figure 2D). Upregulated pathways were involved in ion binding, development, migration, and growth factors, while downregulated pathways were primarily associated with cell signaling, particularly oxygen response, indicating a potential increase in oxidative stress in PDE astrocytes, as has been previously proposed.2,12,36 The ALDH7A1 enzyme has also been described to protect against hyperosmotic stress through the generation of an important cellular osmolyte, formed from betaine aldehyde.37,38 Hyperosmotic stress is coupled to an increase in oxidative stress through generation of reactive oxygen species (ROS) as well as lipid peroxidation (LPO).39 Additionally, ALDH7A1 has been identified to remove several LPO-derived aldehydes that are formed under oxidative conditions, which in turn leads to increased affinity of ALDH7A1 for these toxic aldehydes.37,38 The strong correlation between osmotic and oxidative stress indicates that cytoprotective roles of ALDH7A1 may be 2-fold: (1) producing osmolytes to mitigate osmotic stress and (2) eliminating reactive aldehydes formed due to increased oxidative stress.
Figure 2.
RNA sequencing in PDE patient-derived astrocytes suggests impaired oxygen response and increased oxidative stress
(A) Volcano plot depicting differentially expressed genes (DEGs) between ALDH7A1 KO (KO) and C1 astrocytes. Genes were defined as differentially expressed when the absolute log2 fold change (Log2FC) exceeds 0.58 and with a Benjamini-Hochberg (BH)-adjusted p value below 0.05. Colored dots indicate DEGs; downregulated genes in KO astrocytes are depicted in blue, while upregulated genes are shown in red. The ten genes with the largest difference in expression between KO and C1 astrocytes are indicated. (B) Volcano plot depicting DEGs between astrocytes from P1 and P2 compared to C1 and C2. Genes were defined as differentially expressed when the absolute Log2FC exceeds 0.58 and with a BH-adjusted p value below 0.05. Colored dots indicate DEGs; downregulated genes in KO astrocytes are depicted in light-blue, while upregulated genes are shown in light-red. The ten genes with the largest difference in expression between PDE and control astrocytes are indicated. (C) Venn diagram depicting the number of shared upregulated genes (red) and downregulated genes (blue) between the list of DEGs in KO versus C1 astrocytes and between astrocytes from P1 and P2 versus to C1 and C4. (D) Scatterplot showing the enriched GO terms of the shared genes per ontological category, associated with upregulated (red dots) and downregulated genes (blue dots) of both the DEGs between the KO and C1 astrocytes as well as between the P1 and P2 astrocytes versus C1 and C2 astrocytes. The size of the dots indicates the number of intersected genes between the list of DEGs and the genes associated with the particular term. The p values are indicated as “score” (−log10(p value)).
To explore whether ALDH7A1 deficiency might be linked to oxidative stress, we examined transcriptional differences in LPO-related genes in PDE astrocytes (Figure S3B). LPCAT3 upregulation suggests changes in lipid metabolism that heighten lipid peroxidation vulnerability. Increased expression of TXNRD1, PRDX3, and PRDX1 indicates a response to elevated LPO-related ROS, while PXDN downregulation hampers oxidative stress mitigation. Downregulation of ACSL4, ALOX5, and CHAC1 impacts lipid metabolism and glutathione defenses, reducing the ability to counteract oxidative stress.40,41,42 These findings imply a decreased capacity to manage oxidative stress and a higher risk of LPO damage in PDE astrocytes, though these changes were more pronounced in P1 and the ALDH7A1 KO astrocytes compared to P2.
To directly probe for oxidative stress in PDE astrocytes, we measured 8-hydroxy-2′-deoxyguanosine (8-Oxo-dG) under baseline conditions using immunocytochemistry. 8-Oxo-dG is one of the major DNA oxidation products and therefore generally considered a measure for oxidative stress. We observed a significant increased signal for 8-Oxo-dG in the ALDH7A1 KO astrocytes compared to C1 (Figure 3A). As ALDH7A1 has been predicted to play a role in lipid metabolism, we also measured LPO-related oxidative stress through 4-hydroxynonenal (4-HNE) immunostaining, one of the main metabolites produced during LPO. We observed the 4-HNE fluorescent signal to be significantly increased in the ALDH7A1 KO astrocytes compared to C1 (Figure 3B). These findings are in line with the increased ROS levels measured in the ALDH7A1 KO astrocytes compared to C1 (Figure S4). In contrast to the ALDH7A1 KO astrocytes, we did not observe an increase of the 8-Oxo-dG signal (Figure 3C) in any of the PDE patient astrocyte lines. The 4-HNE signal (Figure 3D) was however significantly elevated in P1 and P2 astrocytes compared to control (C1–C4) astrocytes, while ROS levels were increased in the P3 astrocytes (Figure S4). Overall, PDE astrocytes exhibit elevated signs of oxidative stress levels; however, these levels are more pronounced in ALDH7A1 KO astrocytes compared to PDE patient astrocytes.
Figure 3.
Increased OCRs and oxidative stress levels in PDE astrocytes
(A) Representative images of 8-Oxo-dG immunostaining and FC of mean intensity of 8-Oxo-dG per well relative to average intensity of C1 shown for ALDH7A1 KO (KO) and C1 astrocytes. n = 25/6 for C1; n = 40/6 for KO. (B) Representative images of 4-HNE immunostaining and FC of mean intensity of 4-HNE per well relative to average intensity of C1 shown for KO and C1 astrocytes. n = 33/4 for C1; n = 39/4 for KO. For (A) and (B), statistically significant differences were tested through Mann-Whitney test. (C) Representative images of 8-Oxo-dG immunostaining and FC of mean intensity of 8-Oxo-dG per well relative to average intensity of merged controls shown for P1, P2, P3, and merged controls (C1–C4). n = 75/6 for C1–C4; n = 30/6 for P1; n = 40/6 for P2; n = 32/5 for P3. (D) Representative images of 4-HNE immunostaining and FC of mean intensity of 4-HNE per well relative to average intensity of merged controls shown for P1, P2, P3, and merged controls (C1–C4). n = 66/4 for C1–C4; n = 29/4 for P1; n = 31/4 for P2; n = 39/4 for P3. For (C) and (D), statistically significant differences were tested through Kruskal-Wallis test combined with Dunn’s testing. (E) Fold change (FC) of basal respiration (BR), ATP production (AP), maximal respiration (MR), proton leak (PL), spare capacity (SC), and non-mitochondrial oxygen consumption (NMOC) is depicted for KO and C1 astrocytes at DIV 35, as measured with seahorse assay. For BR, AP, PL, and NMOC, n = 28/2 for C1; n = 29/2 for KO. For MR and CP, n = 14/2 for C1; n = 14/2 for KO. Statistically significant differences were tested through unpaired t test or Mann-Whitney test. (F) FC of BR, AP, MR, PL, SC, and NMOC is depicted for DIV 35 astrocytes from P1, P2, P3, and merged controls (C1 and C2). For BR, AP, PL, and NMOC, n = 56/2 for C1–C4; n = 12/1 for P1; n = 24/2 for P2; n = 24/2 for P3. For MR and CP, n = 28/2 for C1–C4; n = 6/1 for P1; n = 12/2 for P2; n = 12/2 for P3. Statistically significant differences were tested through ordinary one-way ANOVA and Dunnett’s multiple comparison test or with Kruskal-Wallis test combined with Dunn’s multiple comparison test. All data are shown as mean values with standard deviation (mean ± SD). For all graphs in this figure, statistical significance is indicated by ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005, ∗∗∗∗p < 0.0001. Scale bars, 50 μm. Exact p value per condition is provided in Table S1.
The lysine degradation pathway is partially localized within the mitochondria, so the accumulation of toxic lysine intermediates is thought to contribute directly to mitochondrial dysfunction.43,44,45 Furthermore, increased ROS and the resulting oxidative stress are also known to impair mitochondrial function.46,47,48,49 Given these facts and the elevated oxidative stress identified in PDE astrocytes, we further investigated mitochondrial function in these cells. We performed Seahorse assay to measure cellular respiration by monitoring the OCR. We included astrocytes derived from the three patients, the ALDH7A1 KO and its control (C1), and an additional control line (C3). We observed a significant increase for all OCR parameters (Figures 3E and S5) as well as increased intracellular ATP concentration (Figure S4C) in ALDH7A1 KO astrocytes relative to its control, suggesting a compensatory upregulation of mitochondrial activity, likely as an adaptive response to the accumulation of toxic lysine intermediates and the associated metabolic and oxidative stress. Similarly, some of these parameters were significantly elevated in P2 and P3 astrocytes (Figures 3F, S4D, and S5) but not in P1 astrocytes, which instead showed a reduction in proton leak compared to control. Overall, PDE astrocytes exhibited an increased OCR, but in line with oxidative stress measurements, this increase was more pronounced in ALDH7A1 KO astrocytes than in PDE patient astrocytes.
Rescue of the metabolic and cellular phenotypes in ALDH7A1 KO astrocytes by targeting the upstream enzyme AASS
Considering that the accumulation of toxic lysine derivatives in PDE is causative for the neurologic phenotype, therapeutic strategies for PDE should aim to reduce this accumulation by blocking the entrance of lysine into the catabolic pathway. As previously indicated, increased lysine levels due to deficiency in the first step of the lysine pathway catalyzed by AASS have not caused any relevant clinical phenotype.16 Moreover, AASS inhibition to treat other lysine metabolism disorders such as GA1 has already been described20,21 and more recently also for PDE using mouse models.22,23 Therefore, we considered AASS a potential target for therapeutic inhibition to reduce the accumulation of toxic lysine derivatives in PDE. To test this hypothesis, we first generated a double knockout (DKO) hiPSC line for ALDH7A1 and AASS (ALDH7A1/AASS DKO) by targeting AASS through CRISPR-Cas9 genome editing in the ALDH7A1 KO hiPSC line (Figure S6). Western blotting of the ALDH7A1 KO hiPSC line and ALDH7A1/AASS DKO hiPSC line confirmed full KO of ALDH7A1 in both lines and around 95% reduction of AASS levels in the ALDH7A1/AASS DKO hiPSC line compared to C1 (Figure 4A). Notably, we observed that AASS levels were already reduced by approximately 50% in the ALDH7A1 KO hiPSC line compared to C1, suggesting that cells might already downregulate AASS as a homeostatic response to loss of ALDH7A1.
Figure 4.
Rescue of metabolic and cellular phenotypes in PDE astrocytes by the decrease of AASS enzyme
(A) Schematic overview of ALDH7A1 KO (KO) hiPSC line and ALDH7A1/AASS DKO (DKO) hiPSC line, including western blot for AASS, ALDH7A1, and GAPDH proteins. (B) Schematic representation of ALDH7A1 deficiency combined with AASS deficiency in the lysine pathway including the corresponding biomarkers. The normalized intensity of P6C, PA, and AAA measured via NGMS in KO, DKO, and C1 astrocytes is depicted. n = 3 for C1; n = 3 for KO; n = 3 for DKO. Statistically significant differences were tested through ordinary one-way ANOVA and Tukey’s multiple comparison test. (C) Representative images of 8-Oxo-dG immunostaining (scale bars, 50 μm) and FC of mean intensity of 8-Oxo-dG per well relative to average intensity of C1 shown for KO, DKO, and C1 astrocytes. n = 25/6 for C1; n = 40/6 for KO; n = 40/6 for DKO. (D) Representative images of 4-HNE immunostaining (scale bars, 50 μm) and FC of mean intensity of 4-HNE per well relative to average intensity of C1 shown for KO, DKO, and C1 astrocytes. n = 34/4 for C1; n = 39/4 for KO; n = 38/4 for DKO. For both (C) and (D), statistically significant differences were tested through Kruskal-Wallis test combined with Dunn’s testing. (E) Representative images of CellRox assay (scale bars, 50 μm) and FC of mean intensity of CellRox per well relative to average intensity of C1 shown for KO, DKO, and C1 astrocytes. n = 27/4 for C1; n = 33/4 for KO; n = 34/4 for DKO. Statistically significant differences were tested through ordinary one-way ANOVA and Dunnett’s multiple comparison test. (F) Fold change (FC) of basal respiration (BR), ATP production (AP), proton leak (PL), maximal respiration (MR), spare capacity (SC), and non-mitochondrial oxygen consumption (NMOC) is depicted for KO, DKO, and C1 astrocytes at DIV 35. For BR, AP, PL, and NMOC, n = 28/2 for C1; n = 29/2 for KO; n = 27/2 for DKO. For MR and CP, n = 14/2 for C1; n = 14/2 for KO; n = 13/2 for DKO. Statistically significant differences were tested through unpaired t test or Mann-Whitney test. All data are shown as mean values with standard deviation (mean ± SD). For all graphs in this figure, statistical significance is indicated by ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005, ∗∗∗∗p < 0.0001. Exact p value per condition is provided in Table S1. (G) FC of relative ATP concentration through ATP determination kit in C1, KO, and DKO. n = 29/3 for C1; n = 29/3 for KO; n = 29/3 for DKO. Statistically significant differences were tested through unpaired t test.
The ALDH7A1 KO, ALDH7A1/AASS DKO, and C1 lines were differentiated toward astrocytes, all showing expression of the astrocyte markers and no effect on differentiation due to additional KO of AASS (Figures 1E and S1B). To investigate whether targeting AASS in ALDH7A1 KO astrocytes could rescue the metabolic phenotype, we measured the PDE biomarkers under basal conditions using NGMS (Figures 4B and S2). As expected, AAA levels remained low in the ALDH7A1/AASS DKO astrocytes, similar to ALDH7A1 KO astrocytes. P6C and PA levels in the ALDH7A1/AASS DKO astrocytes were significantly reduced but did not reach control levels. This suggests that while targeting AASS in ALDH7A1 KO astrocytes mitigates the metabolic imbalance, it does not fully rescue it. We next assessed whether this partial metabolic restoration was sufficient to reduce oxidative stress in ALDH7A1 KO astrocytes. In the ALDH7A1/AASS DKO astrocytes, 8-Oxo-dG (Figure 4C), 4-HNE (Figure 4D), and ROS levels (Figure 4E) were fully normalized to control levels. This correlation suggests that partial metabolic restoration through AASS targeting may be associated with normalization of oxidative stress responses in ALDH7A1 KO astrocytes.
Finally, we investigated if AASS inhibition could also restore the increased OCR observed in the ALDH7A1 KO astrocytes (Figure 4F). We observed that the proton leak and non-mitochondrial oxygen consumption as well as the intracellular ATP levels (Figure 4G) were fully restored in the ALDH7A1/AASS DKO astrocytes. However, the OCR parameters basal respiration, ATP production, maximal respiration, and spare capacity were not completely normalized. Altogether, these findings indicate that by targeting AASS in PDE astrocytes, both the metabolic phenotype as well as cellular phenotypes, defined by increased oxidative stress and OCR, can be ameliorated.
AON-mediated downregulation of AASS can partially rescue metabolic and cellular phenotypes in PDE patient astrocytes
To recreate a situation comparable to PDE-affected individuals, in which AASS is present during development and therefore can only be targeted after development, we designed an alternative strategy in which we partially reduced AASS levels using antisense technology. Two distinct AON strategies, splice-switching AONs (ssAONs) and gapmers, were employed to target and promote the degradation of AASS transcripts (Figure S7A). ssAONs (S1, S2, and S3) were designed to target a splice site (enhancer) to promote exon skipping, resulting in an out-of-frame transcript and subsequently degradation by nonsense-mediated decay. In contrast, gapmers (G1, G2, and G3; Figure S7B) contain a DNA core sequence flanked by RNA wings that binds to the pre- and mRNA to degrade the transcript through RNase H recruitment. Our AONs were designed to target the region encoding for the LKR domain of AASS (the large AASS isoform) leaving the SDH domain (shortest isoform) intact. We specifically aimed to target the LKR AASS activity, as the accumulation of saccharopine (the product generated by the SDH activity) has been shown to be neurotoxic, while the accumulation of lysine seems to be safe.50
To evaluate the efficiency of our AONs to downregulate AASS, wild-type fibroblasts were transfected with liposomes including the ssAONs (0.5 or 1.0 μM), gapmers (0.2 or 0.5 μM), or the respective negative sense control (Son) corresponding to the ssAONs (SonS; at 1.0 μM) or gapmers (SonG; at 0.5 μM). Four days post delivery, AASS downregulation was assessed at RNA level through quantitative reverse-transcription PCR (RT-qPCR) (Figure S7C) and protein level (Figure S7D) by western blot. For the RT-qPCR, amplicons spanning the region between exons 4–5 and exons 6–8 of the AASS transcript were amplified, as these exons are unique to the large AASS isoform and specifically encode the LKR domain. Although we observed efficient exon skipping upon transfection with the ssAONs, this did not result in degradation of the AASS transcript (Figures S7C and S7D). We therefore excluded the ssAONs from following experiments. Transfection with the gapmers resulted in approximately 50%–80% downregulation of AASS levels relative to SonG control (Figures S7C and S7D). We selected G3 for further validation in astrocytes. Accordingly, 4- to 5-week-old C1 astrocytes were transfected for 7 days with G3 using liposomes at concentrations ranging from 0.05 to 0.5 μM. We observed approximately 50% remaining AASS expression with RT-qPCR (Figure S8A) and around 10% remaining AASS levels with western blotting (Figure S8B) upon G3 delivery at 0.5 μM in C1 astrocytes. Finally, at 0.5 μM, G3 resulted in significant reduction of AASS expression relative to SonG in both the ALDH7A1 KO and PDE patient astrocytes, ranging from 50% to 70% downregulation (Figures 5A and S8C). AASS downregulation of G3 in P3 astrocytes showed ∼50% reduction of AASS levels (Figure 5B).
Figure 5.
Gapmer-mediated rescue of metabolic phenotype and oxygen consumption in P3 astrocytes
All experiments included non-treated control (NT), G3 at 0.5 μM (and 0.05 μM), and a sense oligonucleotide (SonG) control at 0.5 μM. (A) Relative expression of AASS exon 4–5 and exon 6–8 in P3 astrocytes 7 days post G3 delivery, assessed by qPCR and normalized to GUSB. Expression is shown as a percentage relative to SonG. For AASS exon 4–5, n = 6/2 for NT; n = 6/2 for G3 0.05 μM; n = 6/2 for G3 0.5 μM; n = 6/2 for SonG. For AASS exon 6–8, n = 5/3 for NT; n = 7/3 for G3 0.05 μM; n = 7/3 for G3 0.5 μM; n = 7/3 for SonG. Statistical significance was determined by ordinary one-way ANOVA with Dunnett’s multiple comparison test. (B) Semi-quantification of AASS protein levels relative to GAPDH, with representative (cropped) western blot image (n = 3) of P3 astrocytes 7 days post G3 delivery. Statistical significance was tested using one-way ANOVA with Dunnett’s multiple comparison test. (C) Schematic of ALDH7A1 deficiency and G3-mediated AASS downregulation in the lysine pathway, highlighting corresponding biomarkers. Normalized PA intensity and fold change (FC) of P6C and AAA levels via NGMS are presented for NT, G3, and SonG conditions. n = 3 for NT; n = 3 for G3; n = 3 for SonG. Statistical significance was assessed via one-way ANOVA and Tukey’s multiple comparison test. (D and E) Representative immunostaining images (scale bars, 50 μm) and FC of mean intensity per well relative to NT for 8-Oxo-dG (D) and 4-HNE (E) under NT, G3, and SonG conditions. For 8-Oxo-dG immunostaining, n = 13/2 for NT; n = 13/2 for G3; n = 13/2 for SonG. For 4-HNE immunostaining, n = 13/2 for NT; n = 16/2 for G3; n = 15/2 for SonG. Statistical significance was determined via Kruskal-Wallis test with Dunn’s post hoc test. (F) FC values of basal respiration (BR), ATP production (AP), proton leak (PL), maximal respiration (MR), spare capacity (SC), and non-mitochondrial oxygen consumption (NMOC) for NT, G3, and SonG conditions in P3 astrocytes. For BR, AP, PL, and NMOC, n = 29/2 for NT; n = 33/2 for G3; n = 29/2 for SonG. For MR and CP, n = 14/2 for NT; n = 16/2 for G3; n = 16/2 for SonG. Statistical significance was tested using unpaired t test or Mann-Whitney test. All data are shown as mean values with standard deviation (mean ± SD). For all graphs, statistical significance is indicated as ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005, ∗∗∗∗p < 0.0001. Exact p value per condition is provided in Table S1.
We then assessed whether 50% downregulation of AASS in PDE patients could mitigate the metabolic and cellular phenotypes. As the metabolic and OCR parameters were most severely affected in the P3 astrocytes, we used this patient line for the functional validations. The metabolic phenotype was assessed using NGMS 7 days upon G3 delivery (0.5 μM) including non-treated (NT) and SonG control (Figure 5C). As expected, AAA levels were unchanged in the G3-treated P3 astrocytes but still lower compared to control (indicated by the dotted line). Notably, P6C levels were almost normalized to control levels in the G3-treated P3 astrocytes. Although PA levels were improved in the G3-treated condition of the P3 astrocytes, it was not fully normalized to control levels. Other metabolites in pathways related to AASS and lysine metabolism were unaffected by G3 treatment (Figure S9).
We next evaluated oxidative stress levels following treatment with G3 (0.5 μM) for 7 days. Consistent with previous findings, immunostaining for 8-Oxo-dG and 4-HNE did not reveal increased levels of these markers compared to control at baseline (Figures S10A and S1B), and no significant difference was observed between the G3 and control conditions (Figures 5D, 5E, S10C, and S10D). This indicates that when no baseline differences are present, AASS inhibition does not seem to affect oxidative stress levels. However, increased levels of 8-Oxo-dG and 4-HNE in P1 and P2 (Figures S10C and S10D) were also not rescued upon G3 treatment. In addition, the significantly increased ROS levels through CellRox assay in P3 astrocytes (Figure S4) were unchanged upon G3 treatment (Figure S11A). This suggests that G3-mediated downregulation of AASS does not decrease ROS levels in P3 astrocytes, at least under the current conditions.
Lastly, we evaluated the ability of G3 (0.5 μM) to reduce the OCR in P3 astrocytes (Figure 5F). We observed that the basal respiration, ATP production, and non-mitochondrial oxygen consumption were completely normalized to control levels in the P3 astrocytes upon G3 treatment. The proton leak, maximal respiration, and spare capacity (Figure 5F), as well as the intracellular ATP concentration (Figure S11B), were also significantly reduced in the G3 condition compared to the NT condition but reached even lower levels compared to control. The non-mitochondrial oxygen consumption was also significantly reduced in the SonG condition compared to NT, of which the effects and/or toxicity could be either chemistry or sequence dependent.51 Altogether, our data indicate that G3-mediated downregulation of AASS in mature astrocytes is able to partially restore PDE biomarkers and increase OCR.
Discussion
In this study, we used isogenic ALDH7A1 KO hiPSCs and PDE patient-derived hiPSCs, differentiated into astrocytes, to investigate the underlying mechanisms and potential adjunct treatments for PDE. Both ALDH7A1 KO and patient astrocytes showed elevation of PDE biomarkers. Bulk RNA sequencing was used to explore underlying mechanisms, which revealed impaired oxygen response and increased oxidative stress levels. This was further validated by increased markers of oxidative stress including higher levels of ROS and LPO (4-HNE) as well as elevated OCRs in PDE astrocytes. Notably, targeting AASS in PDE astrocytes using CRISPR-Cas9 and AON-mediated downregulation significantly improved the metabolic phenotype and reduced OCRs, highlighting AASS reduction as a promising therapeutic approach for PDE.
Our phenotypic assessment of PDE astrocytes revealed differences between the ALDH7A1 KO and PDE patient astrocytes. We found that ALDH7A1 KO and PDE patient astrocytes showed a similar trend, but these phenotypes were milder in the patients compared to the ALDH7A1 KO astrocytes, similar to what has been described for another IMD.52 A key factor underlying the pronounced difference between the ALDH7A1 KO and PDE patient astrocyte phenotypes is the complete loss of ALDH7A1 expression in the ALDH7A1 KO astrocytes due to the full KO of the gene. In contrast, patients with PDE harbor a missense variant that allows expression of the ALDH7A1 enzyme, probably with some residual activity (Figure S12). We also observed significant differences between the three PDE patient astrocytes. For example, P3 astrocytes showed significantly higher PDE biomarker levels compared to P1 and P2 astrocytes, despite all patients sharing the same mutation, and P2 and P3 being siblings, reducing the possible effects of variability due to genetic background.
In addition to its role in the lysine pathway, ALDH7A1 has been implicated in protecting against oxidative stress through the production of osmolytes as well as by the detoxification of aldehydes produced during LPO.37,38,39 In line with our results, ALDH7A1 deficiency has previously been linked to ROS imbalance and subsequent oxidative stress.36 This could also explain the more pronounced phenotype in the ALDH7A1 KO astrocytes compared to the PDE patient astrocytes. The elevation of 8-Oxo-dG exclusively in ALDH7A1 KO astrocytes, but not in any PDE patient-derived line, suggests that ALDH7A1 primarily protects against DNA oxidation, with a less pronounced role in mitigating LPO-related oxidative stress.
Increased levels of PA have been previously associated with elevated oxidative stress levels through increases in H2O2 for several other metabolic disorders53,54 and have been described to affect antioxidant capacity of the astrocytes.11 In addition, accumulation of PA and P6C,43,44,45 as well as increased oxidative stress,46,47,48,49 is known to contribute to mitochondrial dysfunction. Our findings also show a correlation between elevated PDE biomarkers and dysregulated OCR (either decreased, indicating impaired mitochondrial function, or increased, suggesting potential compensatory upregulation), both of which are indicative of mitochondrial dysfunction. Interestingly, P1 and P2 showed increased 4-HNE but not P3, while P3 astrocytes showed highest levels of PA. However, ALDH7A1 levels were much higher in P3 compared to P1 and P2 (Figure S12). Increased ALDH7A1 levels, and therefore possibly increased antioxidant function, might directly protect against oxidative damage. One contributing factor to explain why ALDH7A1 levels are higher in P3 compared to the other patients (Figure S12) may be patient-specific differential post-transcriptional regulation, such as differences in protein stability, folding, or subcellular localization, which could affect the residual enzymatic activity.55,56 Additionally, patient-specific cellular adaptations or epigenetic differences, even among siblings, could influence lysine metabolism and contribute to the observed variability in biomarker accumulation.57,58 However, technical or biological variability in metabolite measurements (culture conditions, sampling time points, or batch effects) may also contribute to the observed differences and should be considered when interpreting these data. So the exact explanation remains unknown and requires further investigation. Altogether, the interplay between OCR, oxidative stress, and the accumulation of toxic lysine metabolites in PDE appears to be complex and bidirectional, where increased OCR can exacerbate oxidative stress, while oxidative stress and the buildup of metabolites like PA may impair mitochondrial function, leading to altered OCR. These dynamic interactions underscore the need for further investigation to better understand their combined role in disease progression and potential therapy.
One of the limitations of this study is the use of unrelated controls for comparison with PDE patient astrocytes. While these controls provided a necessary baseline, inherent genetic variability between unrelated individuals introduced increased variability in the assays, making it challenging to consistently achieve statistical significance across some parameters. To address this limitation, the inclusion of isogenic controls, such as PDE patient astrocytes with corrected mutations via CRISPR-Cas9, could serve as a more precise comparison. In general, metabolism is highly dynamic,59,60 so the observed differences could also be dependent on the state of the cultured astrocytes. Although we did not observe visual differences between the patient-derived astrocytes, it is possible that the astrocyte cultures have a different developmental trajectory affecting their metabolism.
Similar to the strategy established for GA1, another IMD of the lysine metabolism pathway, AASS modulation has very recently been proposed as a therapeutic approach for PDE using mouse models.22,23 While these studies demonstrated rescue of PDE-associated metabolic deficits in DKO mice (at germline level), here we extend this concept to human model systems, applying more translatable approaches to better reflect patient physiology and possible therapeutic translation. We developed two independent strategies to downregulate AASS: CRISPR-Cas9 genome editing to show proof of concept and a gapmer, which potentially could be translated into a therapy for patients with PDE. The main difference between these two strategies is that, for the ALDH7A1/AASS DKO astrocytes, AASS is not present throughout development and therefore the accumulation of direct byproducts caused by lysine catabolism is minimal. In contrast, in the patient astrocytes, the AASS enzyme and therefore metabolite accumulation are present during development, and AASS can only be inhibited post development. Both approaches showed partial rescue of the PDE biomarkers and rescue of the OCR and ATP levels. In contrast to the P3 astrocytes, which did not exhibit increased oxidative stress at baseline, DNA oxidation, LPO stress, and ROS levels were rescued in the ALDH7A1/AASS DKO astrocytes. The rescue of oxidative stress in ALDH7A1/AASS DKO astrocytes, but not in G3-treated patient astrocytes, suggests that the observed oxidative stress in patient astrocytes might be unrelated to the lysine pathway. However, we cannot rule out that longer treatment durations or higher silencing effect might be necessary to achieve a measurable reduction of oxidative stress markers in PDE patient astrocytes. Still, the effects on the metabolic phenotype and OCR of the ALDH7A1/AASS DKO astrocytes and the G3-treated P3 astrocytes (around 50% remaining AASS protein levels) were relatively similar. This suggests that partial reduction in AASS levels (50%), instead of complete knockdown of AASS, is already sufficient for a partial rescue in PDE patient astrocytes, although there is variability across different PDE patients (Figure S7). A limitation of the present study is that saccharopine could not be reliably quantified with our current metabolomic setup. Consequently, we cannot determine whether AASS downregulation alters saccharopine levels in PDE astrocytes. As saccharopine is a direct metabolite of the lysine pathway, future studies using optimized metabolomic approaches will be required to assess this aspect more conclusively. Here, we present proof of concept for a therapeutic strategy directly translatable to patients with PDE. Future work should extend these findings by validating AASS inhibition in additional human models and by conducting safety and toxicity studies, alongside investigations in animal models where behavioral and seizure outcomes can be assessed, as these aspects were beyond the scope of the present work.
In conclusion, our study suggests that dysregulated OCR and increased oxidative stress contribute to the disease mechanism in PDE. Additionally, we provide evidence that substrate reduction through AASS inhibition could be a potential therapeutic approach for PDE. We demonstrated that partial reduction of AASS levels leads to a partial rescue of PDE biomarkers, elevated OCR, and oxidative stress in PDE astrocytes, offering a promising avenue for future treatment strategies for patients with PDE.
Materials and methods
hiPSC line information
In this study, four independent control lines were used, of which C1, C2, and C3 are commercially available: C1 is derived via episomal reprogramming from fibroblasts of a 30-year-old Japanese male control (GM25256; from Coriell Institute, Camden, USA), C2 is derived from fibroblasts of a healthy 24-year-old female (UMGi020-A; from University Medical Center Goettingen, Goettingen, Germany), and C3 is derived from fibroblasts of a 40-year-old female control (HPSI0314i-hoik_1; from Cambridge BioResource, Cambridge, United Kingdom). C2 and C3 are both reprogrammed using Sendai viral vectors. C4 was generated via lentiviral reprogramming from fibroblasts of a 41-year-old female control.
Diagnosis of patients with PDE was based on both genetic and metabolic screening. All patients presented typical characteristics of PDE at the time of biopsy, and all harbor the c.1279G>C (p.Glu427Gln) variant in ALDH7A1 in homozygosis. P2 and P3 are siblings, minimizing the effect of genetic background. The PDE patient hiPSC lines were created via episomal reprogramming of the patient fibroblasts upon approval by the ethics committee and signed informed consent. Lines were fully validated as described elsewhere.29 In addition, C1 was used to create a full KO of ALDH7A1 using CRISPR-Cas9 editing system. Generation and validation of this line have previously been described.30 Accordingly, the ALDH7A1 KO line was used to create an additional KO of AASS using CRISPR-Cas9 resulting in a homozygous missense variant in intron 4 (c.388-640A>G). Two guide RNAs were designed (TGATACAGCCTTCGAATCGGCGG/TCATAGAGGAGTACGGGTAGTGG) and cloned into pSpCas9(BB)-2A-Puro (PX459) V2.0 (Addgene, #62988), similarly to what has been described for the ALDH7A1 KO line.30
hiPSC-astrocyte differentiation
Astrocytes were differentiated according to a previously described protocol.31 Briefly, hiPSCs were seeded upon dissociation with TrypLE (Gibco; 12604021) onto human recombinant laminin-521 (20 μg/mL; Biolamina; LN521-05 diluted in 1× dPBS++ [Gibco; 14040117]) pre-coated 6-well plates and cultured in astrocyte medium (AM; ScienCell; 1801) supplemented with RevitaCell (Gibco; A2644501) and Primocin at 37°C/5% CO2. The next day, AM supplemented with Primocin was refreshed to remove dead cells and to withdraw RevitaCell from the medium. AM supplemented with Primocin was refreshed every other day, and at 100% confluency, the astrocytes were split by dissociation using TrypLE. Accordingly, the cell pellet was resuspended in AM supplemented with RevitaCell and Primocin, and all cells were transferred into either a T25 flask (Corning; 430372) during the first passage or a T75 flask (Corning; 430641U) during the second passage, followed by full change of AM supplemented with Primocin the day after. Hereafter, the astrocytes were split at 90%–100% confluency at 1:3 onto T75 flasks and cultured in AM with Primocin but in the absence of RevitaCell. After 5 weeks of differentiation, medium changes were performed only twice a week. The cells were imaged using the Invitrogen EVOS XL Core Configured Cell Imager.
hiPSC maintenance
hiPSCs were cultured on Geltrex-coated (Gibco; A1413301) 6-well plates (Corning; 353046) in Essential 8TM Flex Medium kit (Gibco; A2858501) supplemented with Primocin (0.1 μg/mL; InvivoGen; ANT-PM-2) at 37°C/5% CO2. Medium was refreshed every 2 to 3 days, and at around 80% confluency, the hiPSCs were passaged using ReLeSR (STEMCELL Technologies; 100-0483) upon washing in Dulbecco’s phosphate-buffered saline (dPBS; Gibco; 14190169).
Immunocytochemistry
During fixation, the cells were incubated with 4% paraformaldehyde (Sigma-Aldrich; 441244)/4% sucrose (Sigma; S7903) for 15 min and accordingly washed three times with PBS (1×; Sigma; P5493). The cells were blocked for 1 h with blocking buffer (BB; 5% normal donkey serum [Jackson ImmunoResearch; 017-000-121], 5% normal goat serum [Invitrogen; 10189722], 5% normal horse serum [Gibco; 26050070], 0.1% D-lysine [Sigma-Aldrich; L8021], 1% glycine [Sigma-Aldrich; G7126], 1% BSA [Sigma-Aldrich; A-6003], and 0.4% Triton X-100 [Sigma-Aldrich; T8787] in PBS) at room temperature (RT). Primary and secondary antibodies were diluted in BB and incubated at 4°C overnight or at RT for 1 h, respectively. Cells were incubated for 10 min at RT with Hoechst (Thermo Scientific; 62249) diluted in PBS to stain for the nuclei of the cells. Accordingly, cells were mounted using DAKO fluorescent mounting medium (Dako; S3023). The following antibodies were used: rabbit anti-Vimentin (1:300; Abcam; ab92547), rabbit anti-GFAP (1:300; Sigma-Aldrich; AB5804), mouse anti-CD44 (1:300; Invitrogen; MA5-13890), mouse anti-ALDH1L1 (1:300; Novus Biologicals; NBP2-50045), rabbit anti-GLUD1 (1:300; Invitrogen; PA5-28301), mouse anti-8-Oxo-dG (1:500; R&D Systems; 4354-MC-050), mouse anti-4-HNE (1:500; R&D Systems; MAB3249), goat anti-rabbit Alexa Fluor 568 (1:1,000; Invitrogen; A11011), and goat anti-mouse Alexa Fluor 488 (1:1,000; Invitrogen; A11029). Zeiss Axio Imager Z1 was used to image the samples.
NGMS sample preparation and analysis
To measure the PDE biomarkers within the astrocytes, cells were prepared by dissociation using TrypLE, plated onto 12-well plates (Corning; 353043) and cultured in AM at 37°C/5% CO2. One day prior harvesting, AM was fully refreshed. Biological triplicates of each sample were prepared for NGMS analysis according to a previously described protocol.61 Briefly, the astrocytes were washed twice with RT sample buffer, consisting of ammonium carbonate (75 mM; Honeywell; 207861-500G) diluted in Ultrapure water and adjusted pH of 7.2–7.4 using acetic acid. Immediately upon washing with sample buffer, the cells were frozen in liquid nitrogen on the culture plates. Metabolite extraction from the frozen cells was done by 2 min incubation with 250 μL cold extraction buffer (2:2:1 [v/v/v] methanol [Honeywell; 34860–2.5L]:acetonitrile [Biosolve; 0001207802BS]:ultrapure water) followed by another 3 min incubation with new extraction buffer. The two extracts were pooled and centrifuged at 14,000 × g for 3 min at 4°C. Accordingly, the supernatant was dried using a vacuum centrifuge (SpeedVac, Thermo Fisher Scientific), and the samples were stored at −80°C until further use. Metabolites were reconstituted in 80 μL ultrapure water/0.1% HCOOH and analyzed using an ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry (UHPLC-QTOF-MS54). Analyses were performed using an Agilent 1290 UHPLC system coupled to an Agilent 6545 QTOF mass spectrometer, equipped with a dual electrospray ionization source. Each sample was run in duplicate in both negative and positive ionization modes. Two microliters of extracted metabolite sample were injected onto an Acquity HSS T3 (C18, 2.1 × 100 mm, 1.8 μm) column (Waters) operating at 40°C. Each analytical batch included astrocyte samples, analytical quality control samples, and a pooled sample of all astrocyte samples to check for integrity of the automated data analysis pipeline. The biological triplicates of all samples were measured in duplicate. To correct for possible run-order influence on signal intensities, the technical duplicates were analyzed in antiparallel run order. In addition, eight random astrocytes samples were injected at the start of each analytical batch to condition the analytical platform. For analysis, relative peak area was extracted and accordingly normalized for the peak area of phenylalanine.
RNA sequencing
RNA sequencing library preparation
Astrocyte cultures of C1, C4, ALDH7A1 KO, P1, and P2 were prepared for RNA sequencing. Biological triplicates of all astrocyte cultures were seeded onto 6-well plates in AM and cultured at 37°C/5% CO2. At confluency, the astrocytes were washed with ice-cold PBS twice and were then collected using DNA/RNA shield (Zymo Research; #R1200-125). Subsequently, RNA extraction was performed utilizing the Quick-RNA Microprep kit (Zymo Research, #R1051) following the manufacturer’s protocol. The quality of the extracted RNA was evaluated using Agilent’s Tapestation system, with RNA integrity number values falling within the range of 7.3–9.6. Next, cDNA libraries were prepared using the NEBNext Ultra II Directional RNA Library Prep Kit, followed by sequencing of paired-end reads on an Illumina NovaSeq 6000 platform at GenomeScan B.V. Leiden.
RNA sequencing data processing
We used Fastp62 to eliminate PolyG artifacts and clip adapters, including a list of adapter sequences currently used by Illumina. Subsequently, we mapped the reads to the GRCh38 human reference genome using HISAT2. As the library was reversely stranded, we set-rna-strandness = RF. The resulting SAM files were sorted and indexed using SAMtools.63 Next, we performed UMI deduplication with UMI-tools,64 and feature counting was done with Subread’s featureCounts.65
RNA sequencing analysis
Raw count matrices were loaded in R version 4.2.1. When Ensemble IDs mapped to the same gene symbols, we considered only the IDs with the highest expression per sample. Counts belonging to the Y chromosome were excluded. We filtered out lowly expressed genes by keeping genes with expression values higher than 0.5 transcripts per million reads in at least three samples. The remaining counts were then transformed into a DGEList using the DGEList function from edgeR package.66 Subsequently, voom normalization of limma package61 was applied, including normalization factors determined by edgeR’s calcNormFactors. Principal component analysis was conducted on voom normalized and scaled data using prcomp function. Differential expression analysis was carried out for ALDH7A1 KO and Ctrl astrocytes. Additionally, comparisons were made between the patients with PDE and the two control cell lines. For both cases, we normalized using voom and factoring based on condition. Subsequently, limma’s functions lmFit, makeContrasts, contrasts.fit, and eBayes were used to estimate the condition coefficient and its corresponding p value for each gene. Genes were defined as differentially expressed if they exhibited a Benjamini-Hochberg-adjusted p value below 0.05, coupled with a Log2 fold change exceeding 0.58.
Enrichment analysis was separately conducted for DEGs in ALDH7A1 KO vs. control and PDE patients vs. control comparisons, using the go function from the R package gprofiler2 v.0.2.1. To manage redundancy among enriched GO terms (including biological processes, cellular components, or molecular functions), we performed clustering analysis and aggregated terms with high semantic similarity with the functions calculateSimMatrix and reduceSimMatrix from the rrvgo v.1.2.0 R package, setting threshold = 0.7.
Seahorse
OCR was assessed using the Agilent Seahorse XF Cell Mito Stress Test Kit (Seahorse Bioscience). Astrocytes were seeded at a density of 8,000 cells per well for baseline experiments and 4,000 cells per well for gapmer experiments, in AM supplemented with Primocin and RevitaCell at 37°C/5% CO2. The day after plating, AM supplemented with Primocin was fully refreshed to withdraw RevitaCell from the medium. One hour before the assay, AM was replaced with Agilent Seahorse XF Base Medium supplemented with 10 mM glucose, 1 mM sodium pyruvate, and 200 mM L-glutamine, and the cells were incubated at 37°C without CO2. During the recording, basal oxygen consumption was measured four times, followed by three measurements after each addition of 1 μM oligomycin A, 1–4 μM carbonyl cyanide 4-trifluoromethoxy phenylhydrazone (FCCP), 0.5 μM rotenone, and 0.5 μM antimycin A. Each measurement cycle consisted of 3 min of mixing, 3 min of waiting, and 3 min of measuring. The OCR values were normalized for protein concentration to account for differences in cell number and cell size. To determine the protein concentration for each well, the Pierce BCA protein assay was used according to the manufacturer’s instructions (Thermo Scientific 23225). The most optimal FCCP concentration (e.g., maximal respiration) was determined for each line and used for further analysis.
Oxidative stress assay
To measure oxidative stress in astrocytes, immunostainings for both 8-Oxo-dG and 4-HNE were performed. 8-Oxo-dG is an oxidized derivative of deoxyguanosine, one of the major products of DNA oxidation, and therefore considered a measure for oxidative stress.67 4-HNE is an α,β-unsaturated hydroxyalkenal that is produced during LPO in cells. Increased 4-HNE is therefore also associated with increased oxidative stress.68 In addition, ROS levels were assessed using the CellROX Green assay (Thermo Fisher Scientific, C10444). CellROX Green is a cell-permeant fluorogenic dye that is weakly fluorescent in its reduced state but becomes brightly fluorescent and photostable upon oxidation by ROS, thereby representing a readout for oxidative stress. Living astrocytes were incubated with 5 μM probe for 30 min prior to fixation. After fixation, the nuclei were stained with Hoechst, and the cells were imaged within 1 hour. For all assays, the astrocytes were plated either onto glass coverslips (Epredia; 631-0713) or on 96-well culture plates (Greiner; 655090). For baseline characterization, the cells were fixed at 70%–95% confluency, around 3–4 days after seeding. The AON-treated astrocytes were fixated 7 days after AON delivery. Immunostainings for 8-Oxo-dG and 4-HNE were performed as described previously.
ATP determination
Intracellular ATP levels were measured using the ATP Determination Kit (Thermo Fisher Scientific, A22066) according to the manufacturer’s protocol. This bioluminescence-based assay quantifies ATP by measuring the light emitted from the luciferase-catalyzed reaction of luciferin in the presence of ATP. Cells were lysed using a lysis buffer composed of RIPA buffer (pH 7.5; 50 mM Tris-HCl [Invitrogen; 15567027], 150 mM NaCl [Sigma-Aldrich; S5886], 1 mM EDTA [Sigma-Aldrich; 03690], and 1% Triton X-100 in PBS). Luminescence was recorded at 560 nM using a plate reader.
We imaged at a 20× magnification using the Zeiss Axio Imager Z1. To compare signal intensities between different experimental conditions, all conditions within a batch were acquired with the same settings. Fluorescent signals were quantified using Fiji software. The mean intensity per image was calculated and corrected for the total area of the cells in the respective image. The total area was based on the astrocyte-specific cytoskeleton marker vimentin. Fold change (FC) intensity was measured for each well relative to the averaged intensity of the merged controls for baseline characterization or relative to the NT condition for the gapmer experiments.
AON design
The AONs used in this study were designed following the guidelines for splice switching AONs in terms of region accessibility.69 To analyze the accessibility of the target RNA secondary structure, Mfold software version 6.4 was used. The sequence properties, including length, GC content, and Tm of the AONs, were calculated using OligoCalc online software version 3.27. Customized oligonucleotides were purchased from Eurogentec (Liege, Belgium). The gapmer and sense oligonucleotide (SonG) control contained a core DNA sequence of 12 nucleotides with a phosphorothioate (PS) backbone, flanked by 4 RNA-nucleotide LNA/PS wings (G3 sequence: UCCU-GAAGAATGCCAC-CAGC; SonG sequence: CGAC-CACCGUAAGAAG-UCCU). The lyophilized gapmer and SonG were resuspended in sterile PBS to a working concentration of 100 μM. For the other AONs (ssAONs and gapmers) designed in this study, see Table S1.
Gapmer delivery
Astrocytes were seeded onto 12-well culture plates in AM. The day after, the gapmer was delivered at two different concentrations (0.05 and 0.5 μM), whereas the SonG was delivered only at the highest concentration (0.5 μM). One well was kept untreated (NT) in parallel. The gapmer was delivered using FuGENE HD reagent (Promega, Madison, WI, USA) as previously described.65 AM was refreshed every 2 to 3 days (without the gapmer). Seven days after gapmer delivery, the astrocytes were used for seahorse assay, fixated for oxidative stress assays, or collected for RNA analysis or western blot.
RT-qPCR
Total RNA was extracted from the cells using the NucleoSpin RNA Mini Kit (Macherey-Nagel; MN 740955.250) following the manufacturer’s instructions, with the exceptions of omitting β-mercaptoethanol and extending the incubation time with rDNase to 30 min instead of 15 min. Subsequently, the RNA was reverse-transcribed into cDNA utilizing the iScript cDNA Synthesis Kit (Bio-Rad; 1708891). For qPCR, GoTaq qPCR Master Mix (Promega; A6002) was used. The qPCR was conducted on the 7500 Fast Real-Time PCR System (Applied Biosystems) with the following cycling conditions: denaturation at 95°C for 2 min, followed by 40 cycles of 30 s at 95°C and 30 s at 60°C, and a melting curve stage of 15 s at 95°C, 30 s at 60°C, and 15 s at 95°C. All samples were prepared in triplicate, and outliers were identified if a value deviated by more than 0.5 Ct from the other two values within the technical triplicate. The relative mRNA expression was determined using the 2−ΔΔCt method, normalized to the housekeeping gene GUSB. Primers are listed in Table S1.
Western blot
To lyse the cells, the medium was aspirated, and the cells were washed with PBS. Subsequently, lysis buffer (as described previously), supplemented with protease inhibitors (cOmplete Mini; Roche; 11,836,153,001), was added to the cells. Prior to blotting, the protein concentration was determined using Pierce BCA protein assay according to the manufacturer’s instructions (Thermo Scientific; 23227). Subsequently, the samples were loaded with an equal amount of protein (ranging from 5 to 15 μg) onto 4%–15% Mini-PROTEAN TGX Stain-Free Protein Gels (Bio-Rad; 4568084) for protein separation through SDS-PAGE (Bio-Rad; 30 min at 200 V). Accordingly, the separated proteins were transferred to nitrocellulose membranes (Bio-Rad, #1704158) using the Trans-Blot Turbo Transfer System (Bio-Rad; 7 min at 25 V and constant 1.3 A). Membranes were blocked in 5% non-fat milk (Santa-Cruz; sc-2325) in TBS (Millipore; 524750-1EA)/0.1% tween (Merck; 8.22184.0500) (TBS-T) for 1 h at RT and incubated overnight at 4°C with the respective primary antibodies diluted in 5% non-fat milk in TBS-T. The following primary antibodies were used: rabbit anti-AASS (1:1,000; Prestige antibodies; HPA020734-100UL), rabbit anti-ALDH7A1 (1:500; Invitrogen; PA5-54750), and rabbit anti-GAPDH (1:2,000; Cell Signaling Technology, 2118). Horseradish peroxidase-conjugated goat anti-rabbit antibodies (1:50,000; Invitrogen, G21234) were used for visualization.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (version 10 for Windows, GraphPad Software). We first determined whether data were normally distributed using the D’Agostino & Pearson test, Anderson-Darling test, Shapiro-Wilk test, and Kolmogorov-Smirnov test. When only two conditions were compared, unpaired t test was used. When data of only two conditions were not normally distributed, we performed Mann-Whitney test. To test statistical significance for three or more conditions (either different cell lines or treatments), one-way ANOVA combined with Sidak’s multiple comparison tests was used. If these data were not normally distributed, we performed Kruskal-Wallis test combined with Dunn’s testing for multiple comparisons. Data are shown as mean and standard deviation in bar diagrams unless stated otherwise. Results with p values lower than 0.05 were considered as significantly different (∗), p < 0.01 (∗∗), p < 0.001 (∗∗∗), p < 0.0001 (∗∗∗∗). Details about statistics are reported in Table S1.
Data and code availability
The datasets generated during the current study are available from the corresponding author on reasonable request. Source data underlying Figures 1, 2, 3, 4, 5, and S1–S9 are available in Table S1. The GEO accession number for the RNA sequencing data in this paper is GSE287216.
Acknowledgments
We gratefully acknowledge the entire CHARLIE consortium for the constant feedback and support. In particular, we would like to acknowledge Prof. Ron Wevers for insightful discussions about the metabolomic measurements. We would also like to thank Dr. Werner Koopman and Dr. Merel Adjobo-Hermans for their assistance with identifying proper oxidative stress readouts. Furthermore, we want to acknowledge all members of the N.N.K. lab and the Collin & Garanto lab for the continuous help and support.
This study has been initiated by the CHARLIE consortium (EJPRD grant no. 825575 awarded to C.D.M.v.K. and N.N.K.). I.M.E.S. was supported by an internal Radboudumc PhD grant provided by the Radboud Institute for Molecular Life Sciences (awarded to C.D.M.v.K. and A.G.) and the Catalyst Grant form United for Metabolic Diseases (UMD-CG-2023-001), which is financially supported by Metakids (awarded to A.G., I.M.E.S., C.D.M.v.K., and N.N.K.).
Author contributions
C.D.M.v.K., N.N.K., and A.G. conceived and supervised the study. I.M.E.S., C.D.M.v.K., B.R.L., N.N.K., and A.G. (partially) designed the experiments. I.M.E.S., U.E., S.P., R.M., G.-J.S., S.B.v.K., and A.O. performed or assisted during the experiments. C.D.M.v.K., N.N.K., and A.G. provided resources. I.M.E.S., U.E., M.A., S.P., R.M., G.-J.S., S.B.v.K., A.O., H.H.A.-S., D.J.L., C.D.M.v.K., N.N.K., and A.G. performed or assisted during data analysis and interpretation. I.M.E.S., N.N.K., and A.G. wrote the paper. U.E., M.A., S.P., R.M., G.-J.S., S.B.v.K., A.O., D.J.L., B.R.L., and C.D.M.v.K. edited the paper.
Declaration of interests
The antisense molecules used as a possible therapeutic strategy to silence AASS are under evaluation for filing an official patent claim.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.omtn.2025.102728.
Supplemental information
References
- 1.Coughlin C.R., 2nd, Swanson M.A., Spector E., Meeks N.J.L., Kronquist K.E., Aslamy M., Wempe M.F., van Karnebeek C.D.M., Gospe S.M., Jr., Aziz V.G., et al. The genotypic spectrum of ALDH7A1 mutations resulting in pyridoxine dependent epilepsy: A common epileptic encephalopathy. J. Inherit. Metab. Dis. 2019;42:353–361. doi: 10.1002/jimd.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Shekaili H.H., Petkau T.L., Pena I., Lengyell T.C., Verhoeven-Duif N.M., Ciapaite J., Bosma M., van Faassen M., Kema I.P., Horvath G., et al. A novel mouse model for pyridoxine-dependent epilepsy due to antiquitin deficiency. Hum. Mol. Genet. 2020;29:3266–3284. doi: 10.1093/hmg/ddaa202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stockler S., Plecko B., Gospe S.M., Jr., Coulter-Mackie M., Connolly M., van Karnebeek C., Mercimek-Mahmutoglu S., Hartmann H., Scharer G., Struijs E., et al. Pyridoxine dependent epilepsy and antiquitin deficiency: clinical and molecular characteristics and recommendations for diagnosis, treatment and follow-up. Mol. Genet. Metab. 2011;104:48–60. doi: 10.1016/j.ymgme.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 4.Mills P.B., Struys E., Jakobs C., Plecko B., Baxter P., Baumgartner M., Willemsen M.A.A.P., Omran H., Tacke U., Uhlenberg B., et al. Mutations in antiquitin in individuals with pyridoxine-dependent seizures. Nat. Med. 2006;12:307–309. doi: 10.1038/nm1366. [DOI] [PubMed] [Google Scholar]
- 5.van Karnebeek C.D.M., Tiebout S.A., Niermeijer J., Poll-The B.T., Ghani A., Coughlin C.R., 2nd, Van Hove J.L.K., Richter J.W., Christen H.J., Gallagher R., et al. Pyridoxine-Dependent Epilepsy: An Expanding Clinical Spectrum. Pediatr. Neurol. 2016;59:6–12. doi: 10.1016/j.pediatrneurol.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Ngo H.P.T., Nguyen D.Q., Park H., Park Y.S., Kwak K., Kim T., Lee J.H., Cho K.S., Kang L.W. Conformational change of organic cofactor PLP is essential for catalysis in PLP-dependent enzymes. BMB Rep. 2022;55:439–446. doi: 10.5483/BMBRep.2022.55.9.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jansen L.A., Hevner R.F., Roden W.H., Hahn S.H., Jung S., Gospe S.M., Jr. Glial localization of antiquitin: implications for pyridoxine-dependent epilepsy. Ann. Neurol. 2014;75:22–32. doi: 10.1002/ana.24027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu J., Qin D., Liang Z., Liu Q., Wang M., Guo Y., Guo W. Dysregulation of astrocyte-derived matrix gla protein impairs dendritic spine development in pyridoxine-dependent epilepsy. Mol. Ther. 2025;33:1785–1802. doi: 10.1016/j.ymthe.2025.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cellini B., Zelante T., Dindo M., Bellet M.M., Renga G., Romani L., Costantini C. Pyridoxal 5'-Phosphate-Dependent Enzymes at the Crossroads of Host-Microbe Tryptophan Metabolism. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21165823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma S., Prasad A.N. Inborn Errors of Metabolism and Epilepsy: Current Understanding, Diagnosis, and Treatment Approaches. Int. J. Mol. Sci. 2017;18 doi: 10.3390/ijms18071384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rho J.M., Boison D. The metabolic basis of epilepsy. Nat. Rev. Neurol. 2022;18:333–347. doi: 10.1038/s41582-022-00651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtis R., Coughlin S.M.G. Pyridoxine-dependent epilepsy: Current perspectives andquestions for future research. Ann. Child Neurol. Soc. 2023;1:24–37. doi: 10.1002/cns3.20016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu B., Du H., Rutkowski R., Gartner A., Wang X. LAAT-1 is the lysosomal lysine/arginine transporter that maintains amino acid homeostasis. Science. 2012;337:351–354. doi: 10.1126/science.1220281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coughlin C.R., Tseng L.A., Bok L.A., Hartmann H., Footitt E., Striano P., Tabarki B.M., Lunsing R.J., Stockler-Ipsiroglu S., Gordon S., et al. Association Between Lysine Reduction Therapies and Cognitive Outcomes in Patients With Pyridoxine-Dependent Epilepsy. Neurology. 2022;99:e2627–e2636. doi: 10.1212/WNL.0000000000201222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santra S., Baumann U. Experience of nitisinone for the pharmacological treatment of hereditary tyrosinaemia type 1. Expert Opin. Pharmacother. 2008;9:1229–1236. doi: 10.1517/14656566.9.7.1229. [DOI] [PubMed] [Google Scholar]
- 16.Houten S.M., Te Brinke H., Denis S., Ruiter J.P., Knegt A.C., de Klerk J.B., Augoustides-Savvopoulou P., Häberle J., Baumgartner M.R., Coşkun T., et al. Genetic basis of hyperlysinemia. Orphanet J. Rare Dis. 2013;8:57. doi: 10.1186/1750-1172-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dancis J., Hutzler J., Ampola M.G., Shih V.E., van Gelderen H.H., Kirby L.T., Woody N.C. The prognosis of hyperlysinemia: an interim report. Am. J. Hum. Genet. 1983;35:438–442. [PMC free article] [PubMed] [Google Scholar]
- 18.Yeganeh M., Auray-Blais C., Maranda B., Sabovic A., DeVita R.J., Lazarus M.B., Houten S.M. A case of hyperlysinemia identified by urine newborn screening. JIMD Rep. 2023;64:440–445. doi: 10.1002/jmd2.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou J., Wang X., Wang M., Chang Y., Zhang F., Ban Z., Tang R., Gan Q., Wu S., Guo Y., et al. The lysine catabolite saccharopine impairs development by disrupting mitochondrial homeostasis. J. Cell Biol. 2019;218:580–597. doi: 10.1083/jcb.201807204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barzi M., Johnson C.G., Chen T., Rodriguiz R.M., Hemmingsen M., Gonzalez T.J., Rosales A., Beasley J., Peck C.K., Ma Y., et al. Rescue of glutaric aciduria type I in mice by liver-directed therapies. Sci. Transl. Med. 2023;15 doi: 10.1126/scitranslmed.adf4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leandro J., Dodatko T., DeVita R.J., Chen H., Stauffer B., Yu C., Houten S.M. Deletion of 2-aminoadipic semialdehyde synthase limits metabolite accumulation in cell and mouse models for glutaric aciduria type 1. J. Inherit. Metab. Dis. 2020;43:1154–1164. doi: 10.1002/jimd.12276. [DOI] [PubMed] [Google Scholar]
- 22.Liang Z., Wu J., Liu Q., Qin D., Wang M., Zhong X., Guo W. Targeting lysine alpha-ketoglutarate reductase to treat pyridoxine-dependent epilepsy. J. Neurosci. 2025;45 doi: 10.1523/JNEUROSCI.0370-25.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Karnebeek C.D.M., Gailus-Durner V., Engelke U.F., Seisenberger C., Marschall S., Dragano N.R.V., da Silva-Buttkus P., Leuchtenberger S., Fuchs H., Hrabě de Angelis M., et al. New treatment for PDE-ALDH7A1: first proof-of-principle of upstream enzyme inhibition in the mouse. Brain Commun. 2025 doi: 10.1093/braincomms/fcaf397. fcaf397. [DOI] [Google Scholar]
- 24.Hammond S.M., Wood M.J.A. Genetic therapies for RNA mis-splicing diseases. Trends Genet. 2011;27:196–205. doi: 10.1016/j.tig.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Hammond S.M., Aartsma-Rus A., Alves S., Borgos S.E., Buijsen R.A.M., Collin R.W.J., Covello G., Denti M.A., Desviat L.R., Echevarría L., et al. Delivery of oligonucleotide-based therapeutics: challenges and opportunities. EMBO Mol. Med. 2021;13 doi: 10.15252/emmm.202013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pena I.A., Roussel Y., Daniel K., Mongeon K., Johnstone D., Weinschutz Mendes H., Bosma M., Saxena V., Lepage N., Chakraborty P., et al. Pyridoxine-Dependent Epilepsy in Zebrafish Caused by Aldh7a1 Deficiency. Genetics. 2017;207:1501–1518. doi: 10.1534/genetics.117.300137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox T.C. Utility and limitations of animal models for the functional validation of human sequence variants. Mol. Genet. Genomic Med. 2015;3:375–382. doi: 10.1002/mgg3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson N.B., Krieger K., Khan F.M., Huffman W., Chang M., Naik A., Yongle R., Hameed I., Krieger K., Girardi L.N., Gaudino M. The current state of animal models in research: A review. Int. J. Surg. 2019;72:9–13. doi: 10.1016/j.ijsu.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 29.Schuurmans I.M.E., van Karnebeek C.D.M., Hoogendoorn A.D.M., Nadif Kasri N., Garanto A. Generation of hiPSC lines from four pyridoxine-dependent epilepsy (PDE) patients carrying the variant c.1279G>C in ALDH7A1 in homozygosis. Stem Cell Res. 2024;79 doi: 10.1016/j.scr.2024.103480. [DOI] [PubMed] [Google Scholar]
- 30.Schuurmans I.M.E., Wu K.M., van Karnebeek C.D.M., Nadif Kasri N., Garanto A. Generation of an induced pluripotent stem cell line carrying biallelic deletions (SCTCi019-B) in ALDH7A1 using CRISPR/Cas9. Stem Cell Res. 2023;71 doi: 10.1016/j.scr.2023.103173. [DOI] [PubMed] [Google Scholar]
- 31.Schuurmans I.M.E., Mordelt A., Linda K., Puvogel S., Duineveld D., Hommersom M.P., Rahm L., Dyke E., Scholten G.-J., Knorz C., et al. Navigating Human Astrocyte Differentiation: Direct and Rapid one-step Differentiation of Induced Pluripotent Stem Cells to Functional Astrocytes Supporting Neuronal Network development. bioRxiv. 2024 doi: 10.1101/2024.03.27.586938. Preprint at. [DOI] [Google Scholar]
- 32.Engelke U.F., van Outersterp R.E., Merx J., van Geenen F.A., van Rooij A., Berden G., Huigen M.C., Kluijtmans L.A., Peters T.M., Al-Shekaili H.H., et al. Untargeted metabolomics and infrared ion spectroscopy identify biomarkers for pyridoxine-dependent epilepsy. J. Clin. Investig. 2021;131 doi: 10.1172/JCI148272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bok L.A., Struys E., Willemsen M.A.A.P., Been J.V., Jakobs C. Pyridoxine-dependent seizures in Dutch patients: diagnosis by elevated urinary alpha-aminoadipic semialdehyde levels. Arch. Dis. Child. 2007;92:687–689. doi: 10.1136/adc.2006.103192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Struys E.A., Bok L.A., Emal D., Houterman S., Willemsen M.A., Jakobs C. The measurement of urinary Delta(1)-piperideine-6-carboxylate, the alter ego of alpha-aminoadipic semialdehyde, in Antiquitin deficiency. J. Inherit. Metab. Dis. 2012;35:909–916. doi: 10.1007/s10545-011-9443-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Plecko B., Paul K., Paschke E., Stoeckler-Ipsiroglu S., Struys E., Jakobs C., Hartmann H., Luecke T., di Capua M., Korenke C., et al. Biochemical and molecular characterization of 18 patients with pyridoxine-dependent epilepsy and mutations of the antiquitin (ALDH7A1) gene. Hum. Mutat. 2007;28:19–26. doi: 10.1002/humu.20433. [DOI] [PubMed] [Google Scholar]
- 36.Yazdani M., Elgstøen K.B.P. Is oxidative stress an overlooked player in pyridoxine-dependent epilepsy? A focused review. Seizure. 2021;91:369–373. doi: 10.1016/j.seizure.2021.07.014. [DOI] [PubMed] [Google Scholar]
- 37.Brocker C., Lassen N., Estey T., Pappa A., Cantore M., Orlova V.V., Chavakis T., Kavanagh K.L., Oppermann U., Vasiliou V. Aldehyde dehydrogenase 7A1 (ALDH7A1) is a novel enzyme involved in cellular defense against hyperosmotic stress. J. Biol. Chem. 2010;285:18452–18463. doi: 10.1074/jbc.M109.077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brocker C., Cantore M., Failli P., Vasiliou V. Aldehyde dehydrogenase 7A1 (ALDH7A1) attenuates reactive aldehyde and oxidative stress induced cytotoxicity. Chem. Biol. Interact. 2011;191:269–277. doi: 10.1016/j.cbi.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martiniere A., Fiche J.B., Smokvarska M., Mari S., Alcon C., Dumont X., Hematy K., Jaillais Y., Nollmann M., Maurel C. Osmotic Stress Activates Two Reactive Oxygen Species Pathways with Distinct Effects on Protein Nanodomains and Diffusion. Plant Physiol. 2019;179:1581–1593. doi: 10.1104/pp.18.01065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao D., Zheng J., Li Z., Yu Y., Chen Z., Wang Q. ACSL4 inhibition prevents macrophage ferroptosis and alleviates fibrosis in bleomycin-induced systemic sclerosis model. Arthritis Res. Ther. 2023;25:212. doi: 10.1186/s13075-023-03190-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu T., Xu X., Li J., Bai M., Zhu W., Liu Y., Liu S., Zhao Z., Li T., Jiang N., et al. ALOX5 deficiency contributes to bladder cancer progression by mediating ferroptosis escape. Cell Death Dis. 2023;14:800. doi: 10.1038/s41419-023-06333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Y., Wu D., Fu Q., Hao S., Gu Y., Zhao W., Chen S., Sheng F., Xu Y., Chen Z., Yao K. CHAC1 as a Novel Contributor of Ferroptosis in Retinal Pigment Epithelial Cells with Oxidative Damage. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms24021582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arruda P., Barreto P. Lysine Catabolism Through the Saccharopine Pathway: Enzymes and Intermediates Involved in Plant Responses to Abiotic and Biotic Stress. Front. Plant Sci. 2020;11:587. doi: 10.3389/fpls.2020.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wempe M.F., Kumar A., Kumar V., Choi Y.J., Swanson M.A., Friederich M.W., Hyland K., Yue W.W., Van Hove J.L.K., Coughlin C.R. Identification of a novel biomarker for pyridoxine-dependent epilepsy: Implications for newborn screening. J. Inherit. Metab. Dis. 2019;42:565–574. doi: 10.1002/jimd.12059. [DOI] [PubMed] [Google Scholar]
- 45.Natarajan S.K., Muthukrishnan E., Khalimonchuk O., Mott J.L., Becker D.F. Evidence for Pipecolate Oxidase in Mediating Protection Against Hydrogen Peroxide Stress. J. Cell. Biochem. 2017;118:1678–1688. doi: 10.1002/jcb.25825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zong Y., Li H., Liao P., Chen L., Pan Y., Zheng Y., Zhang C., Liu D., Zheng M., Gao J. Mitochondrial dysfunction: mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024;9:124. doi: 10.1038/s41392-024-01839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kowalczyk P., Sulejczak D., Kleczkowska P., Bukowska-Ośko I., Kucia M., Popiel M., Wietrak E., Kramkowski K., Wrzosek K., Kaczyńska K. Mitochondrial Oxidative Stress-A Causative Factor and Therapeutic Target in Many Diseases. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222413384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi E.H., Kim M.H., Park S.J. Targeting Mitochondrial Dysfunction and Reactive Oxygen Species for Neurodegenerative Disease Treatment. Int. J. Mol. Sci. 2024;25 doi: 10.3390/ijms25147952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guo C., Sun L., Chen X., Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013;8:2003–2014. doi: 10.3969/j.issn.1673-5374.2013.21.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo Y., Wu J., Wang M., Wang X., Jian Y., Yang C., Guo W. The Metabolite Saccharopine Impairs Neuronal Development by Inhibiting the Neurotrophic Function of Glucose-6-Phosphate Isomerase. J. Neurosci. 2022;42:2631–2646. doi: 10.1523/JNEUROSCI.1459-21.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Godfrey C., Desviat L.R., Smedsrød B., Piétri-Rouxel F., Denti M.A., Disterer P., Lorain S., Nogales-Gadea G., Sardone V., Anwar R., et al. Delivery is key: lessons learnt from developing splice-switching antisense therapies. EMBO Mol. Med. 2017;9:545–557. doi: 10.15252/emmm.201607199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parveen Parasar N.K., Poisson L.M., Singh J. iPSC-derived astrocytes to model phenotype-specific differential neuroinflammatory and metabolic responses in X-linked adrenoleukodystrophy. bioRxiv. 2023 doi: 10.1101/2022.09.09.507263. Preprint at. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dalazen G.R., Terra M., Jacques C.E.D., Coelho J.G., Freitas R., Mazzola P.N., Dutra-Filho C.S. Pipecolic acid induces oxidative stress in vitro in cerebral cortex of young rats and the protective role of lipoic acid. Metab. Brain Dis. 2014;29:175–183. doi: 10.1007/s11011-013-9466-3. [DOI] [PubMed] [Google Scholar]
- 54.Wang P., Luo Q., Yang W., Ahammed G.J., Ding S., Chen X., Wang J., Xia X., Shi K. A Novel Role of Pipecolic Acid Biosynthetic Pathway in Drought Tolerance through the Antioxidant System in Tomato. Antioxidants. 2021;10 doi: 10.3390/antiox10121923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chua B.A., Van Der Werf I., Jamieson C., Signer R.A.J. Post-Transcriptional Regulation of Homeostatic, Stressed, and Malignant Stem Cells. Cell Stem Cell. 2020;26:138–159. doi: 10.1016/j.stem.2020.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y., Yi Y., Lv J., Gao X., Yu Y., Babu S.S., Bruno I., Zhao D., Xia B., Peng W., et al. Low RNA stability signifies increased post-transcriptional regulation of cell identity genes. Nucleic Acids Res. 2023;51:6020–6038. doi: 10.1093/nar/gkad300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martin G.M. Epigenetic drift in aging identical twins. Proc. Natl. Acad. Sci. USA. 2005;102:10413–10414. doi: 10.1073/pnas.0504743102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Poulsen P., Esteller M., Vaag A., Fraga M.F. The epigenetic basis of twin discordance in age-related diseases. Pediatr. Res. 2007;61:38R–42R. doi: 10.1203/pdr.0b013e31803c7b98. [DOI] [PubMed] [Google Scholar]
- 59.Smith R.L., Soeters M.R., Wüst R.C.I., Houtkooper R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocr. Rev. 2018;39:489–517. doi: 10.1210/er.2017-00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Carthew R.W. Gene Regulation and Cellular Metabolism: An Essential Partnership. Trends Genet. 2021;37:389–400. doi: 10.1016/j.tig.2020.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Coene K.L.M., Kluijtmans L.A.J., van der Heeft E., Engelke U.F.H., de Boer S., Hoegen B., Kwast H.J.T., van de Vorst M., Huigen M.C.D.G., Keularts I.M.L.W., et al. Next-generation metabolic screening: targeted and untargeted metabolomics for the diagnosis of inborn errors of metabolism in individual patients. J. Inherit. Metab. Dis. 2018;41:337–353. doi: 10.1007/s10545-017-0131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen S., Zhou Y., Chen Y., Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li H., Handsaker B., Wysoker A., Fennell T., Ruan J., Homer N., Marth G., Abecasis G., Durbin R., 1000 Genome Project Data Processing Subgroup The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith T., Heger A., Sudbery I. UMI-tools: modeling sequencing errors in Unique Molecular Identifiers to improve quantification accuracy. Genome Res. 2017;27:491–499. doi: 10.1101/gr.209601.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liao Y., Smyth G.K., Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30:923–930. doi: 10.1093/bioinformatics/btt656. [DOI] [PubMed] [Google Scholar]
- 66.Robinson M.D., McCarthy D.J., Smyth G.K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiorcea-Paquim A.M. 8-oxoguanine and 8-oxodeoxyguanosine Biomarkers of Oxidative DNA Damage: A Review on HPLC-ECD Determination. Molecules. 2022;27 doi: 10.3390/molecules27051620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eskelinen M., Saimanen I., Koskela R., Holopainen A., Selander T., Eskelinen M. Plasma Concentration of the Lipid Peroxidation (LP) Biomarker 4-Etaydroxynonenal (4-HNE) in Benign and Cancer Patients. In Vivo (Athens) 2022;36:773–779. doi: 10.21873/invivo.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garanto A., Collin R.W.J. Design and In Vitro Use of Antisense Oligonucleotides to Correct Pre-mRNA Splicing Defects in Inherited Retinal Dystrophies. Methods Mol. Biol. 2018;1715:61–78. doi: 10.1007/978-1-4939-7522-8_5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request. Source data underlying Figures 1, 2, 3, 4, 5, and S1–S9 are available in Table S1. The GEO accession number for the RNA sequencing data in this paper is GSE287216.