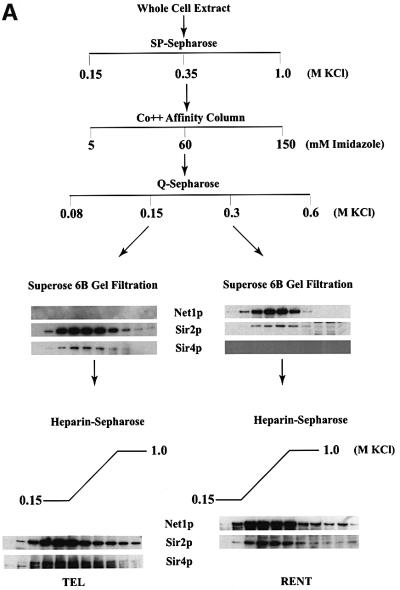
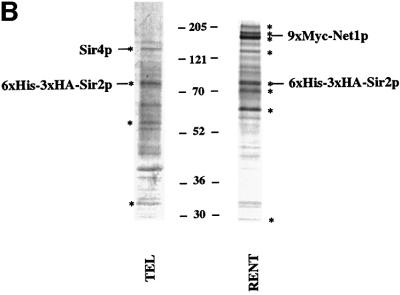
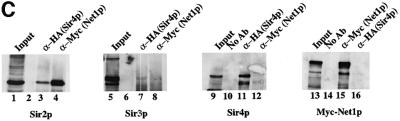
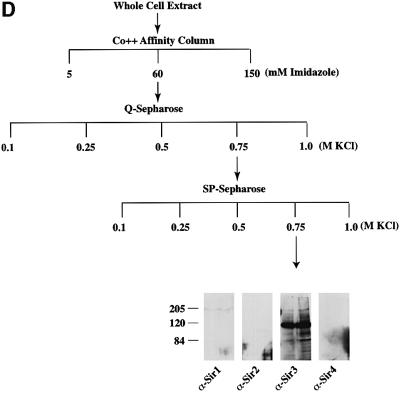
Fig. 2. Purification of Sir protein complexes from yeast whole cell extracts. (A) The fractionation scheme of Sir2p containing complexes from yeast whole cell extracts is summarized in the flow chart. The immunoblots depict selected fractions from the columns probed with antisera against Sir2p, Sir4p and Net1p. (B) Polypeptide composition of the TEL and RENT complexes. Aliquots of the peak fractions from the heparin-Sepharose column were resolved on a 3–18% SDS–polyacrylamide gel and stained with silver. The asterisks denote bands that co-fractionate with Sir2p. Identities of Sir2p, Sir4p and Net1p were ascertained by protein immunoblotting with antibodies specific to these proteins. (C) Co-immunoprecipitation analysis of Sir4p and Net1p. Whole cell extracts (2 mg) generated from ROY 1914 were subjected to immunoprecipitation with commercial antibodies against the Myc and HA epitopes. The input extract as well as aliquots of the immunoprecipitated proteins were resolved on an SDS–polyacrylamide gel and analyzed by protein immunoblotting with antibodies that recognize Sir2p (lanes 1–4), Sir3p (lanes 5–8), Sir4p (lanes 9–12) and Net1p (lanes 13–16). (D) The fractionation scheme of Sir3p containing complexes from yeast whole cell extracts is summarized in the flow chart. The immunoblots depict selected fractions from the SP-Sepharose column probed with antisera against Sir1p, Sir2p Sir3p and Sir4p.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.