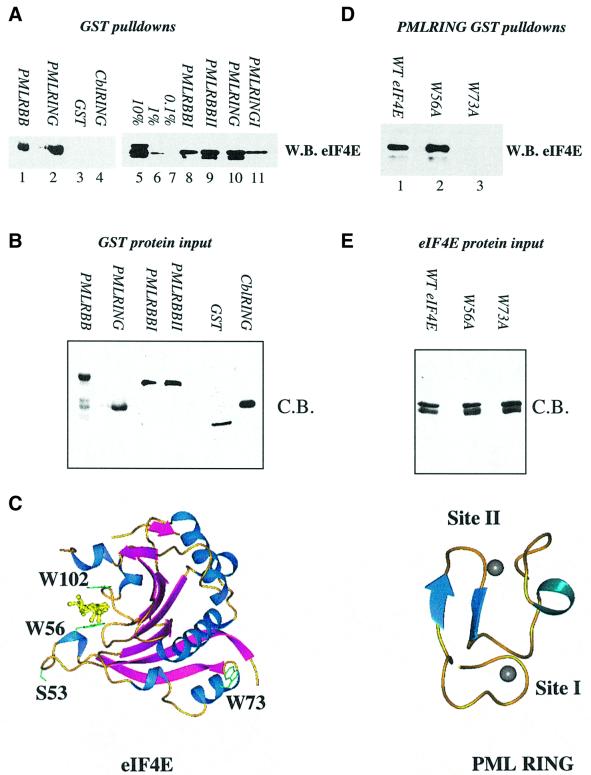
Fig. 1. Purified PML and eIF4E directly interact. (A and D) GST pull-down analysis of PML and eIF4E. Wild-type eIF4E (A) or mutant eIF4E (D) were incubated with GST fusion proteins as indicated. (B and E) Coomassie Blue-stained SDS–PAGE demonstrating production and purity of the mutant proteins used in (A) and (D). In all cases, proteins of the predicted molecular weight were produced. Further, identical quantities of each protein were used in the pull-down assays shown in (A) and (D). For each pull-down, fusion proteins attached to glutathione beads were resolved in the absence of bait proteins to demonstrate that there was no cross-reaction of these proteins with the antibodies used (data not shown). W.B., western blot; C.B., Coomassie Blue-stained SDS–PAGE. RBB indicates that constructs contained the RING and double B-box of PML; I and II indicate targeted mutation of either zinc site I or zinc site II. 10, 1 and 0.1% represent eIF4E input for (A). The lower of the two bands present in lanes 5, 9 and 10 of (A) and in (D) represents a minor degradation product of eIF4E, which is present in some eIF4E protein preparations and is still able to bind PML RING. (C) The three-dimensional structures of eIF4E and PML are shown (1ej1 and 1bor; Borden et al., 1995; Marcotrigiano et al., 1997). Arrows indicate β-sheets; coils, α-helix; balls, zinc atoms. The m7GpppG dinucleotide substrate of eIF4E is shown in yellow. Structures were rendered in PREPI (S.Islam and M.Sternberg).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.