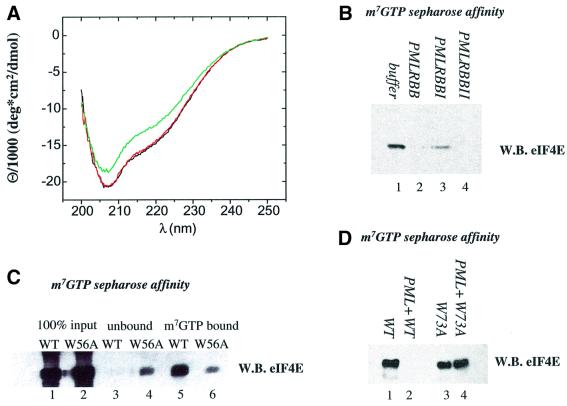
Fig. 6. (A) m7GpppG-binding dramatically affects the conformation of the eIF4E protein. Far-UV CD spectra of bacterially purified eIF4E at 0.24 µM (black), in complex with 3 µM GpppG (red) and with 3 µM m7GpppG (green). (B) PML dramatically reduces the affinity of eIF4E for m7GTP-Sepharose. Bacterially expressed and purified PML RBB or mutants in site I (PMLRBBI) or site II (PMLRBBII) of the RING were mixed with purified eIF4E and applied to m7GTP-Sepharose beads. (C) The W56A mutation reduces the affinity of eIF4E for m7GTP-Sepharose. The affinity of wild-type and W56A mutant of eIF4E was monitored by m7GTP-Sepharose affinity chromatography. The input for each protein preparation is shown, as is the material that bound m7GTP-Sepharose (lanes 5 and 6) and the unbound fraction (lanes 3 and 4). (D) PML does not reduce the affinity of the W73A eIF4E mutant for m7GTP-Sepharose. Results were monitored by western analysis using eIF4E mAb where WB indicates western blot.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.