FIGURE 2.
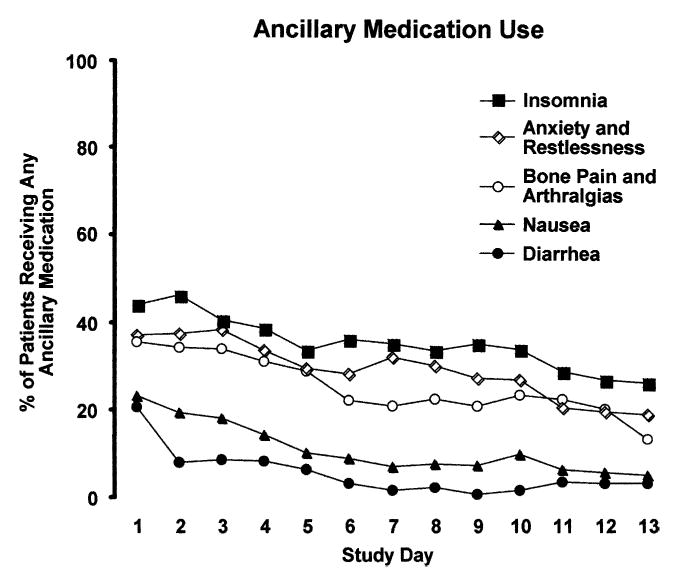
Percent of randomized patients who received a dose of buprenorphine-naloxone and any type of ancillary medication on each day of the dose taper for Insomnia (closed square); Anxiety and Restlessness (open diamond); Bone Pain and Arthralgias (open circle); Nausea (closed triangle); or Diarrhea (closed circle).
