Fig. 1.
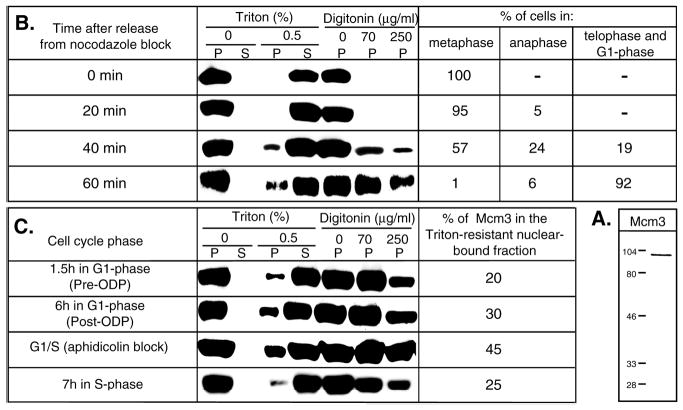
Cell cycle-regulated association of hamster Mcm3 with chromatin. (A) Western blot of total CHOC 400 cellular protein extract probed with an Mcm3-specific antibody. Positions of molecular weight standards (indicated in kDa) are marked on the left. (B,C) Variations in the amount of chromatin-bound Mcm3 during different stages of the cell cycle in CHOC 400 cells. Synchronized cell populations were resuspended in cytoskeleton buffer (for Triton extractions) or transport buffer (for digitonin permeabilization) and incubated for 5 minutes on ice with or without addition of permeabilizing agents as described in Materials and Methods. The cellular or nuclear pellets (P) were separated from the soluble fractions (S) by centrifugation. The proteins from each fraction (only the pellets were analyzed in the case of digitonin extractions) were separated by electrophoresis in 10% SDS-polyacrylamide gels (amounts corresponding to 5×105 cells were loaded in each lane), transferred to nylon membranes and probed with an anti-Mcm3 antibody. In B, the percentages of cells in different stages of mitosis, determined microscopically after staining aliquots of the synchronized cells with 0.1 μg/ml 4′, 6-diamidino-2-phenylindole (DAPI), are indicated on the right for each time point. In C, the relative amounts of chromatin-bound Mcm3 proteins at each time point analyzed were estimated by comparing serial dilutions of the soluble fractions run in parallel to an aliquot of the Triton-resistant fraction and are indicated on the right.
