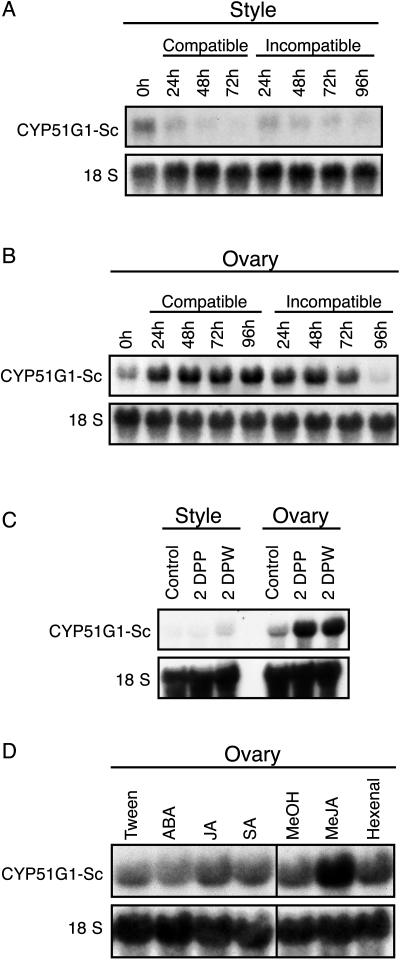
Figure 3.
RNA expression analysis of CYP51G1-Sc transcript levels in styles and ovaries following pollination and stress treatments. A and B, Effect of compatible and incompatible pollination on CYP51G1-Sc transcript levels. CYP51G1-Sc transcript levels were determined by RNA gel-blot analysis in unpollinated pistils on anthesis day (0 h) and in pistils collected from 24 to 96 h after a fully compatible (S11S13 × S12S14) pollination or a fully incompatible (S12S14 × S12S14) pollination. Styles and ovary mRNAs were extracted and blotted separately. C, CYP51G1-Sc transcript levels were determined by RNA gel-blot analysis of tissues 2 DPP or 2 d after wounding (DPW). Styles were either compatibly pollinated or slightly crushed with tweezers and collected 2 d later. Unpollinated and unwounded styles and ovaries served as controls. Ovaries from the same pistils were also collected either 2 DPP or 2 d after wounding. D, CYP51G1-Sc transcript levels were determined by RNA gel-blot analysis of unpollinated ovary tissues collected 48 h after various hormonal treatments. Spray treatments: abscisic acid (ABA) and jasmonic acid (JA) as 50 μm solutions in 0.05% Tween; salicylic acid (SA) as a 5 mm solution in 0.05% Tween. Flowers sprayed with a 0.05% Tween solution served as control. For treatment with volatiles, methyl jasmonate (MeJA) or trans-2-hexenal (Hexenal) were first diluted to 0.1 m in ice-cold methanol, and 100 μL of the compound (equivalent to 10 μL/L of air space) was added to a piece of Whatman paper suspended over the plant in an airtight container. Treatment with 100 μL of methanol (MeOH) served as control. For all RNA gel blots, 10 μg of total RNA from the various tissues was probed with the CYP51G1-Sc cDNA insert. To ascertain equal loading conditions, all RNA gel blots were stripped and reprobed with an 18S ribosomal cDNA probe from S. chacoense.