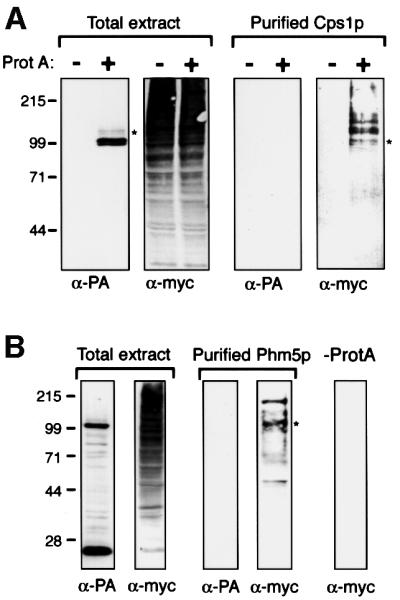
Fig. 7. Ubiquitylation of Cps1p and Phm5p. (A) Δdoa4 cells expressing myc-tagged ubiquitin and either protein A-tagged Cps1p, or only endogenous Cps1p, were processed in parallel. Total extracts, and protein purified by binding to IgG–Sepharose followed by cleavage with TEV protease to release Cps1p from the protein A tag, were immunoblotted with anti-protein A (PA) or anti-myc. Note that protein A is absent from the purified material. Cps1p migrates as a doublet, due to variable glycosylation (Spormann et al., 1992). The faint bands above the protein A chimera are likely to be ubiquitylated forms, but these are under-represented in total cell extracts due to de-ubiquitylation during sample preparation. (B) As (A), but cells expressed protein A-tagged Phm5p. The lower protein A-containing band results from processing close to the transmembrane domain (see Figure 4). In addition, some more random degradation was detectable, which accounts for the smaller myc-tagged proteins in the purified fraction. In both panels, the asterisks indicate the approximate mobility expected for a mono-ubiquitylated protein.
