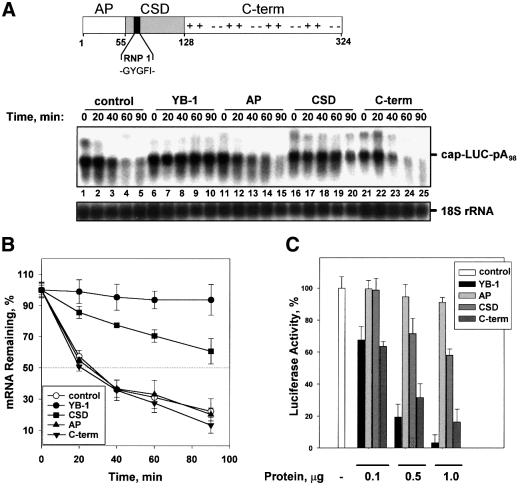
Fig. 4. The cold-shock domain of YB-1 confers mRNA stability, but not translational repression. (A) Top: schematic representation of YB-1 functional domains. The N-terminal AP-rich domain, the central CSD containing RNP 1 consensus motif, and the C-terminal domain with alternating basic (+) and acidic (–) amino acid clusters are indicated. Amino acids are numbered according to Evdokimova et al. (1995). (A) Bottom: effect of YB-1 fragments on mRNA stability. Capped and polyadenylated LUC mRNA (0.1 µg) was incubated in a rabbit reticulocyte lysate with buffer (control) or in the presence of 0.5 µg of YB-1 or its fragments. Total RNA was isolated at the times indicated and LUC mRNA and 18S rRNA were detected by northern blot hybridization with the corresponding probes. (B) Kinetic analysis of LUC mRNA decay. The amounts of LUC mRNA from three independent experiments similar to that shown in (A) were quantified by PhosphorImaging software and normalized to 18S rRNA. (C) Effect of YB-1 fragments on mRNA translation. Capped and polyadenylated LUC mRNA (0.1 µg) was incubated in a rabbit reticulocyte lysate with buffer alone (–) or increasing amounts of the protein indicated. Translation reactions were incubated at 30°C for 60 min and luciferase activity was monitored by luminometer. The luciferase activity in the presence of buffer alone (control) was set as 100%. Error bars denote the standard error from three independent experiments.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.