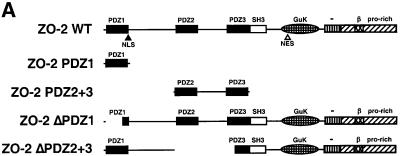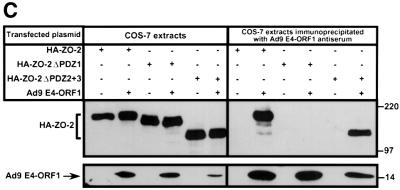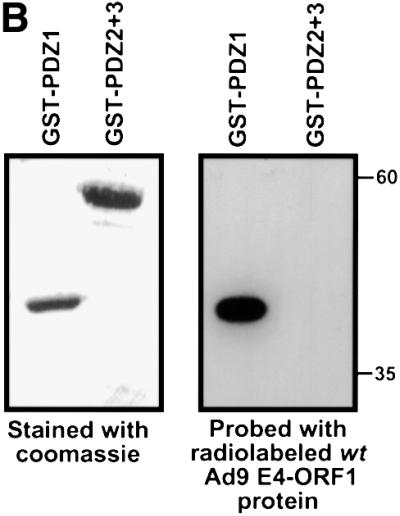
Fig. 4. ZO-2 PDZ1 mediates binding of ZO-2 to Ad9 E4-ORF1. (A) Illustration of ZO-2 protein fragments and ZO-2 deletion mutants. (B) Specific binding of Ad9 E4-ORF1 to ZO-2 PDZ1 in protein blotting assays. Approximately 5 µg of the indicated ZO-2 GST fusion protein, separated by SDS–PAGE and immobilized on a membrane, was either stained with Coomassie Blue dye to verify the presence of equivalent amounts of protein (left panel) or probed with a radiolabeled wild-type Ad9 E4-ORF1 protein probe (right panel). (C) Requirement of ZO-2 PDZ1 for ZO-2 to co-immunoprecipitate with Ad9 E4-ORF1 from cell extracts. Protein (400 µg) from RIPA buffer-lysed COS-7 cells co-transfected with 5 µg of either empty GW1 plasmid or GW1 plasmid expressing Ad9 E4-ORF1 and 5 µg of GW1 plasmid expressing wild-type HA–ZO-2 or mutant HA–ZO-2 lacking either PDZ1 (HA–ZO-2ΔPDZ1) or both PDZ2 and PDZ3 (HA–ZO-2ΔPDZ2+3) was immunoprecipitated with Ad9 E4-ORF1 antibodies. Recovered proteins were separated by SDS–PAGE and immunoblotted with anti-HA or anti-Ad9 E4-ORF1 antibodies. As a control, one-fifteenth the amount of protein used in the immuno precipitation reactions was also directly immunoblotted with the same antibodies.