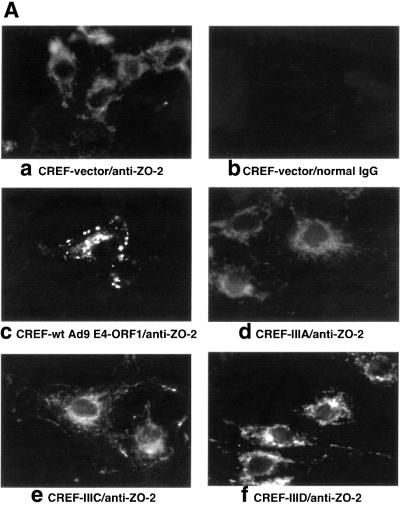
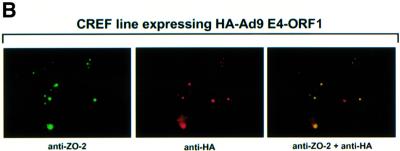
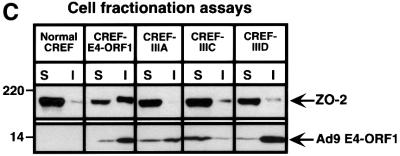
Fig. 5. Ad9 E4-ORF1 aberrantly sequesters ZO-2 in the cytoplasm of CREF cells. (A) Distribution of ZO-2 in normal CREF cells or CREF cells stably expressing either wild-type or the indicated mutant Ad9 E4-ORF1. IF assays were performed with either affinity-purified ZO-2 antibodies (a, c–f) or normal rabbit IgG (b) and visualized by fluorescence microscopy. (B) ZO-2 and Ad9 E4-ORF1 co-localize within cytoplasmic punctate bodies in CREF cells. Double-label IF assays were performed using both affinity-purified ZO-2 and anti-HA antibodies in CREF cells stably expressing HA–Ad9 E4-ORF1. Each of the three panels represents the same field of five cells stained for ZO-2 (left panel), HA–Ad9 E4-ORF1 (center panel) or the merged images (right panel). (C) Ad9 E4-ORF1 aberrantly sequesters ZO-2 within detergent-insoluble complexes in CREF cells. Normal CREF cells or CREF cells stably expressing either wild-type or the indicated mutant Ad9 E4-ORF1 were lysed in RIPA buffer, and extracts were centrifuged to produce RIPA buffer-soluble (S) supernatant and RIPA buffer-insoluble (I) pellet fractions. Protein (100 µg) from S fractions or an equivalent amount from I fractions (see Materials and methods) was separated by SDS–PAGE and immunoblotted with either ZO-2 or Ad9 E4-ORF1 antiserum.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.