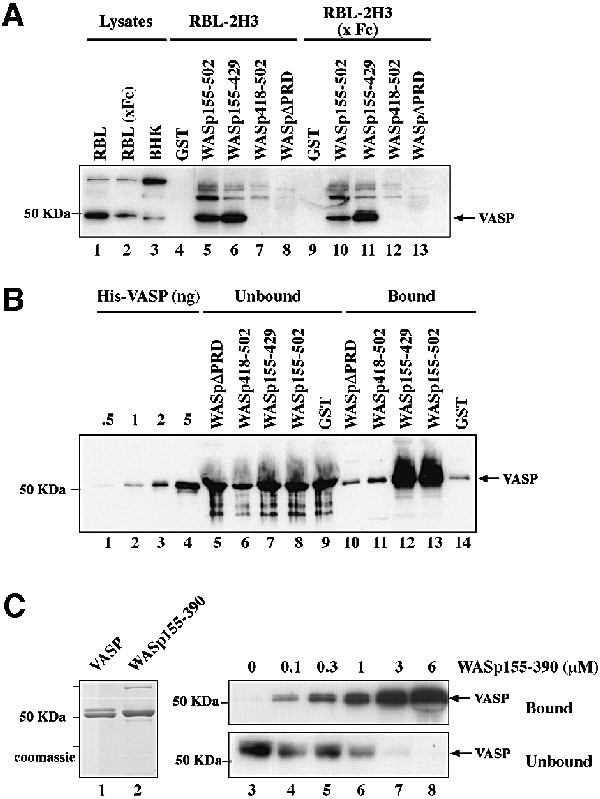
Fig. 6. The PRD of WASp mediates direct interaction with VASP. (A) VASP pulldown from RBL-2H3 cell lysates using the GST–WASp fusion proteins indicated. GST, GST–WASp155–502, –WASp155–429, –WASp418–502 (VCA domain) or –WASpΔPRD (WASp155–502 deleted of the PRD, position 310–390) immobilized on glutathione–Sepharose beads was incubated with cell lysates prepared from unstimulated RBL-2H3 cells, or cells stimulated by cross-linking of FcεRI (x Fc). Bound material was subjected to western blotting with anti-VASP antibodies. Lanes 1–3: total cell lysates; lanes 4–8: GST pulldown from RBL-2H3 cell lysate; lanes 9–13: GST pulldown from lysate of RBL-2H3 cells activated by FcεRI cross-linking. (B) The central PRD of WASp mediates direct binding to VASP. GST or GST–WASp fusion proteins (1 µg) bound to glutathione–Sepharose beads were incubated with recombinant His6–VASP (250 nM) and bound material was subjected to western blotting with anti-VASP antibodies. Lanes 1–4: the indicated amount of His6–VASP; lanes 5–9: unbound material (2% of total); lanes 10–14: bound material (50% of total). (C) Estimation of the equilibrium binding constant of VASP with WASp155–390. His6–VASP or GST–WASp(155–390)His6 (1 µg each) was visualized by Coomassie Blue staining of the gel (lanes 1 and 2). VASP (50 nM) was incubated in the presence of increasing concentrations of WASp155–390 as indicated, in the presence of a fixed amount of glutathione–Sepharose beads. Bound and unbound proteins were subjected to SDS–PAGE followed by western blotting with anti-VASP antibody (lanes 3–8). A value of ∼1 µM was derived for the equilibrium binding constant for VASP with WASp155–390. The results shown are representative of four independent experiments with two different preparations of recombinant proteins.
