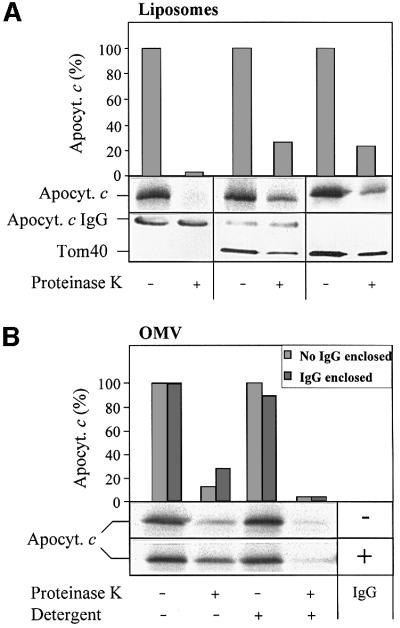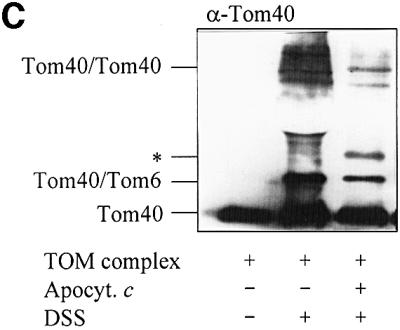
Fig. 5. The TOM complex is sufficient for membrane translocation of apocytochrome c. (A) Liposomes with or without reconstituted TOM complex and enclosed IgG were incubated with radiolabelled apocytochrome c; import was performed and analysed as described in Figure 2. (B) Purified OMV (15 µg/sample) with and without enclosed anti-apocytochrome c IgG were incubated with radiolabelled apocytochrome c and an import reaction was performed. Half the samples were lysed with Triton X-100 and import was analysed as in Figure 2. (C) Crosslinking of apocytochrome c with the TOM complex. Isolated TOM complex (6 µg) was incubated with or without apocytochrome c (10 µg) in the presence or absence of 0.25 mM DSS for 30 min on ice. The reaction was stopped by the addition of 5 mM Tris–HCl pH 7.0. The proteins were precipitated with TCA, separated by SDS–PAGE, blotted on to nitrocellulose and immunostained with anti-Tom40 antiserum. The crosslinking products are indicated (see Rapaport et al., 1998a). The asterisk indicates the crosslinking adduct between apocytochrome c and Tom40. The apparent molecular mass of this crosslinked product (∼52 kDa) fits well to an adduct between these two proteins. We tried to confirm the identity of apocytochrome c by immunostaining. However, the presence of a cross-reacting band (possibly a tetramer of apocytochrome c) in this molecular mass region (even in the absence of TOM complex) made this analysis impossible (data not shown).