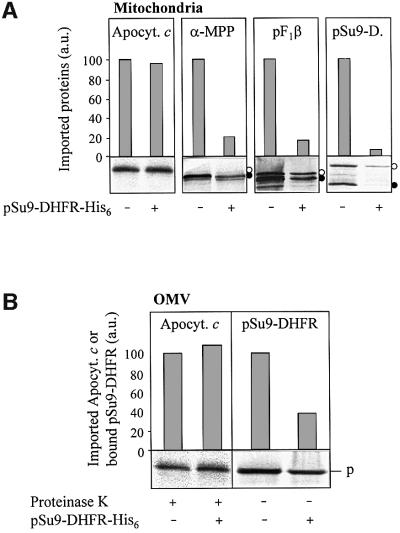
Fig. 6. Chemical amounts of pSu9-DHFR-His6 do not inhibit apocytochrome c translocation. (A) Isolated mitochondria (50 µg/sample) or (B) purified OMV (15 µg/sample) were incubated with radiolabelled apocytochrome c, the precursors (open circles in the autoradiographs) of matrix processing peptidase α-subunit (α-MPP), F1-ATPase β-subunit (pF1β) or Su9-DHFR as indicated, in the presence or absence of chemical amounts of pSu9-DHFR-His6 (4 µM final concentration; Künkele et al., 1998a). After 6 min at 25°C, mitochondria and OMV were re-isolated by centrifugation. Following proteinase K treatment (for apocytochrome c), the proteins were precipitated with TCA, analysed by SDS–PAGE, blotted on to nitrocellulose and subjected to phosphorimager analysis and autoradiography. Import into mitochondria (A) was estimated from the amounts of protease-resistant apocytochrome c and of the processed (mature) forms of the preproteins (closed circles). The signal in the absence of added pSu9-DHFR-His6 was set to 100. Translocation of the presequence of pSu9-DHFR in OMV (B) was taken from the amount of bound preprotein after pelleting the membranes in the presence of 100 mM NaCl (trans site binding; Mayer et al., 1995c). p, precursor; a.u., arbitrary units.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.