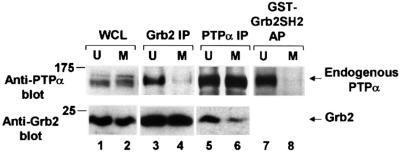
Fig. 4. Decreased in vivo binding of Grb2 to PTPα during mitosis. The association of endogenous PTPα with Grb2 in unsynchronized and mitotic cells was measured by complementary co-immunoprecipitation experiments and by Grb2 SH2 domain affinity-precipitation experiments. Immunoprecipitates made with either anti-Grb2 (lanes 3 and 4) or -PTPα (lanes 5 and 6) antibody and lysates from unsynchronized (U) or mitotic (M) non-overexpressor cells were analyzed by 10% SDS–PAGE and immunoblotted with anti-PTPα or -Grb2 antibody as indicated. Lanes 7 and 8: PTPα was affinity-precipitated from lysates from unsynchronized or mitotic cells using GST–Grb2 SH2 domain fusion protein bound to Sepharose beads and immunoblotted with anti-PTPα antibody only. Direct immunoblots of the whole cell lysates used in lanes 5 and 6 (lanes 1 and 2, bottom panel) or lanes 7 and 8 (lanes 1 and 2, top panel) are also shown. To optimize detection, the different experiments used lysates containing differing amounts of total cell protein: lanes 1 and 2, top, 0.05 mg; lanes 1 and 2 bottom, 0.025 mg; lanes 3 and 4, 0.4 mg; lanes 5 and 6, 1.5 mg; lanes 7 and 8, 1 mg. The positions of molecular weight standards are indicated in kDa.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.