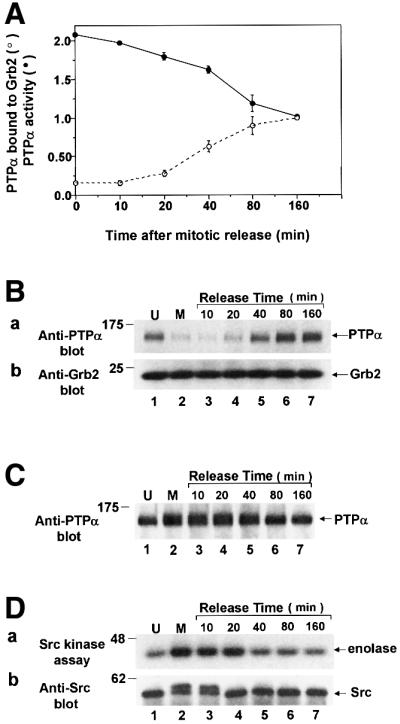
Fig. 7. PTPα tyrosine phosphatase activity and Grb2 binding following release from mitotic arrest. Mitotic non-overexpressor cells were collected after arrest with nocodazole and were then incubated for the indicated amounts of time in normal medium without nocodazole. Cell lysates were then prepared and analyzed for PTPα activity, binding to Grb2 in vivo, electrophoretic mobility and Src activity. (A) The ability of immunoprecipitated endogenous PTPα to dephosphorylate [32P]pTyr-containing MBP was measured as described in Figure 1. The amount of 32P released per molecule of PTPα normalized to the amount released by overexpressed PTPα after 160 min is shown (filled circles). The binding of PTPα to Grb2 was measured in experiments like the one shown in Figure 7B. The amount of PTPα bound normalized to the amount bound after 160 min release is shown (open circles). Error bars indicate the SEM (two experiments). (B) Grb2 was immunoprecipitated from the cell lysates and the amount of co-immunoprecipitated endogenous PTPα was revealed by anti-PTPα immunoblotting (panel a). Immunoblotting of the lower portion of the blot with anti-Grb2 antibody showed that there was no change in the level of Grb2 throughout the release period (panel b). Experimental procedures were as in Figure 4. (C) Anti-PTPα immunoblot of immunoprecipitated endogenous PTPα. (D) Endogenous Src was immunoprecipitated from the cell lysates and its ability to phosphorylate enolase was measured as described in Figure 3A. Aliquots of each immunoprecipitate were immunoblotted with anti-Src antibody (panel b). The positions of molecular weight standards are indicated in kDa.
