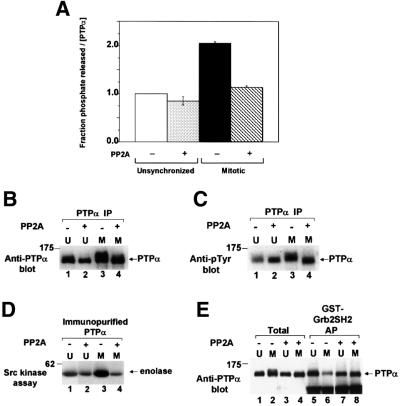
Fig. 8. Effect of serine-dephosphorylation on phosphatase activity and Grb2 binding of PTPα from unsynchronized and mitotic cells. Wild-type PTPα-HA was immunoprecipitated with anti-HA antibody from unsynchronized (U) or mitotic (M) overexpressor cells, either treated (+) or not treated (–) with serine/threonine phosphatase PP2A, and then assayed. (A) The ability of the immunoprecipitates to dephosphorylate [32P]pTyr-containing MBP was assayed as in Figure 1A. Error bars indicate the SEM. (B) Anti-PTPα immunoblot in 10% SDS–PAGE of the immunoprecipitates. (C) Anti-pTyr immunoblot in 10% SDS–PAGE of the immunoprecipitates. The slightly decreased level of tyrosine phosphorylation in unsynchronized untreated cells was not routinely reproducible. (D) The treated and untreated PTPα was eluted and assayed for its ability to dephosphorylate and active immunoprecipitated overexpressed Src as in Figure 2. (E) The treated and untreated PTPα was eluted and affinity-precipitated by GST–Grb2 SH2 domain fusion protein, analyzed by 9% SDS–PAGE and immunoblotted with anti-PTPα antibody (lanes 5–8) as in Figure 5B. For comparison, lanes 1–4 (Total) each contain 40% of the amount of eluant which was affinity-precipitated. The positions of molecular weight standards are indicated in kDa.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.