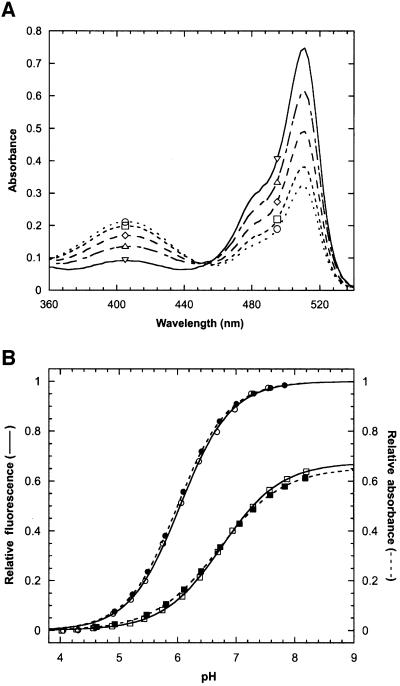
Fig. 4. Redox state-dependent spectral properties of rxYFP149202 at 30°C. (A) Absorption spectra were recorded after equilibration of the protein in redox buffers with [GSH]2/[GSSG] ratios of 0.004 M (open circles), 0.55 M (open squares), 2.17 M (open diamonds) and 8.13 M (open triangles) (see Figure 3A). Reduced protein (open inverted triangles) was obtained by incubation with 10 mM DTT for 2 h. (B) The relative fluorescence (open symbols) and relative absorbance (closed symbols) of reduced (circles) and oxidized (squares) rxYFP149202 as a function of pH. Data were fitted as described in Materials and methods. The estimated values of the chromophore pKa and the Hill coefficient (n) are given in the text.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.