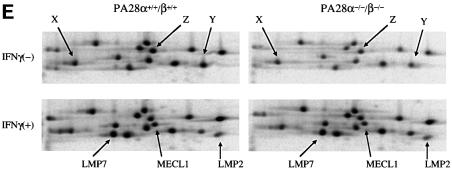
Fig. 3. ATP-dependent protein degradation activity, peptide hydrolysis activities and immunoproteasome formation in wild-type and PA28α–/–/β–/– cells. (A) ATP- and AZ-dependent degradation of [35S]ODC was assayed using crude extracts from wild-type and knockout MEFs cultured with (filled bars) or without (open bars) IFN-γ for 72 h. The results are the mean of determinations performed in triplicate. Error bars represent one standard deviation on each side of the mean. There are statistically significant differences between wild-type and PA28α–/–/β–/– cells (P <0.01, shown as asterisks). The experiment was repeated three times and consistently yielded statistically significant differences between wild-type and knockout MEFs (data not shown). (B) Sedimentation velocity analysis. Samples (2 mg of proteins) from wild-type (open circles) and knockout (filled circles) MEFs were fractionated by glycerol density gradient centrifugation (10–40% glycerol from fraction 1 to fraction 30). Aliquots (20 µl) of individual fractions were used for assay of [35S]ODC degradation activities. Western blot analysis of each fraction was performed using antibodies against X, LMP2 and PA28α. Asterisks indicate artifact bands. Numbers correspond to fraction numbers in the upper and lower panels. (C) Peptide hydrolysis activities. Aliquots of individual fractions prepared in (B) were subjected to peptide hydrolysis assays using three kinds of substrates. Suc-LLVY-AMC and Cbz-LLE-AMC were hydrolyzed in the presence of 0.05% SDS, whereas Boc-LRR-AMC was hydrolyzed without SDS. Open circles, wild-type; filled circles, knockout. (D) Initial assembly of immunoproteasomes. MEFs were cultured in the presence of IFN-γ. After the indicated times (hours), MEFs were harvested for western blot analysis with anti-LMP2 and anti-X antibodies. (E) Two-dimensional gel electrophoresis. MEFs were cultured in the presence or absence of IFN-γ for 48 h, and then metabolically labeled for 4 h followed by a 16 h chase. Cell lysates were immunoprecipitated with anti-20S proteasome antibodies and subjected to isoelectric focusing followed by SDS–PAGE.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.